nab-Sirolimus for Patients With Malignant Perivascular Epithelioid Cell Tumors
- PMID: 34637337
- PMCID: PMC8601264
- DOI: 10.1200/JCO.21.01728
nab-Sirolimus for Patients With Malignant Perivascular Epithelioid Cell Tumors
Erratum in
-
Erratum: nab-Sirolimus for Patients With Malignant Perivascular Epithelioid Cell Tumors.J Clin Oncol. 2023 Dec 10;41(35):5477. doi: 10.1200/JCO.23.02173. Epub 2023 Oct 27. J Clin Oncol. 2023. PMID: 37890128 Free PMC article. No abstract available.
Abstract
Purpose: Malignant perivascular epithelioid cell tumor (PEComa) is a rare aggressive sarcoma, with no approved treatment. To our knowledge, this phase II, single-arm, registration trial is the first prospective clinical trial in this disease, investigating the safety and efficacy of the mammalian target of rapamycin inhibitor nab-sirolimus (AMPECT, NCT02494570).
Patients and methods: Patients with malignant PEComa were treated with nab-sirolimus 100 mg/m2 intravenously once weekly for 2 weeks in 3-week cycles. The primary end point was objective response rate evaluated by independent radiology review. Key secondary end points included duration of response, progression-free survival, and safety. A key exploratory end point was tumor biomarker analysis.
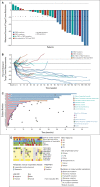
Results: Thirty-four patients were treated (safety evaluable), and 31 were evaluable for efficacy. The overall response rate was 39% (12 of 31; 95% CI, 22 to 58) with one complete and 11 partial responses, 52% (16 of 31) of patients had stable disease, and 10% (3 of 31) had progressive disease. Responses were of rapid onset (67% by week 6) and durable. Median duration of response was not reached after a median follow-up for response of 2.5 years, with 7 of 12 responders with treatment ongoing (range, 5.6-47.2+ months). Twenty-five of 31 patients had tumor mutation profiling: 8 of 9 (89%) patients with a TSC2 mutation achieved a confirmed response versus 2 of 16 (13%) without TSC2 mutation (P < .001). The median progression-free survival was 10.6 months (95% CI, 5.5 months to not reached), and the median overall survival was 40.8 months (95% CI, 22.2 months to not reached). Most treatment-related adverse events were grade 1 or 2 and were manageable for long-term treatment. No grade ≥ 4 treatment-related events occurred.
Conclusion: nab-Sirolimus is active in patients with malignant PEComa. The response rate, durability of response, disease control rate, and safety profile support that nab-sirolimus represents an important new treatment option for this disease.
Conflict of interest statement
Figures


References
-
- Doyle LA, Argani P, Hornick JL: PEComa, in WHO Classification of Tumors Editorial Board. Soft Tissue and Bone Tumours. Lyon, France, International Agency for Research on Cancer, 2020, pp 312-314
-
- Folpe AL, Kwiatkowski DJ: Perivascular epithelioid cell neoplasms: Pathology and pathogenesis. Hum Pathol 41:1-15, 2010 - PubMed
-
- Sanfilippo R, Jones RL, Blay JY, et al. : Role of chemotherapy, VEGFR inhibitors, and mTOR inhibitors in advanced perivascular epithelioid cell tumors (PEComas). Clin Cancer Res 25:5295-5300, 2019 - PubMed
-
- Benson C, Vitfell-Rasmussen J, Maruzzo M, et al. : A retrospective study of patients with malignant PEComa receiving treatment with sirolimus or temsirolimus: The Royal Marsden Hospital experience. Anticancer Res 34:3663-3668, 2014 - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

