Safety and Immunogenicity of a Respiratory Syncytial Virus Prefusion F Vaccine When Coadministered With a Tetanus, Diphtheria, and Acellular Pertussis Vaccine
- PMID: 34637519
- PMCID: PMC9200146
- DOI: 10.1093/infdis/jiab505
Safety and Immunogenicity of a Respiratory Syncytial Virus Prefusion F Vaccine When Coadministered With a Tetanus, Diphtheria, and Acellular Pertussis Vaccine
Abstract
Background: Prevention of respiratory syncytial virus (RSV) disease in infants is an unmet vaccine need, and maternal immunization is a potential strategy to address this need. This study evaluated concomitant administration of RSV stabilized prefusion F subunit vaccine (RSVpreF) and tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccine adsorbed (Tdap) in healthy, nonpregnant women 18‒49 years of age.
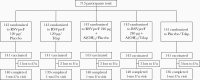
Methods: In this phase 2b, multicenter, placebo-controlled, observer-blind, noninferiority study, participants were randomized to receive RSVpreF in a range of doses and formulations with Tdap or alone, or Tdap alone. Safety and immunogenicity were assessed.
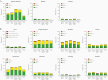
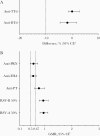
Results: Local reactions and systemic events were generally similar across vaccine groups. Noninferiority of anti-RSV-A and anti-RSV-B immune responses induced by RSVpreF with Tdap was demonstrated compared to RSVpreF alone. Noninferiority of anti-diphtheria toxoid and anti-tetanus toxoid immune responses after administration of RSVpreF with Tdap was demonstrated compared to Tdap alone; noninferiority was not met for anti-pertussis component responses.
Conclusions: RSVpreF was safe and well tolerated when administered with Tdap or alone in nonpregnant women 18‒49 years of age. Immune responses induced by Tdap administered with RSVpreF were noninferior for the tetanus and diphtheria components of Tdap, but not for pertussis.
Clinical trials registration: NCT04071158.
Keywords: RSV vaccine; Tdap vaccine; immunogenicity; maternal immunization; respiratory syncytial virus; safety.
© The Author(s) 2021. Published by Oxford University Press for the Infectious Diseases Society of America.
Figures
Similar articles
-
Safety and immunogenicity of SIIPL Tdap, a new tetanus toxoid, reduced diphtheria toxoid and acellular pertussis vaccine, in healthy subjects 4-65 years of age: A Phase II/III randomized, observer-blinded, active controlled, multicenter clinical study in Germany.Vaccine. 2023 Nov 2;41(46):6810-6819. doi: 10.1016/j.vaccine.2023.09.060. Epub 2023 Oct 10. Vaccine. 2023. PMID: 37827966 Clinical Trial.
-
Safety and Immunogenicity of 3 Formulations of an Investigational Respiratory Syncytial Virus Vaccine in Nonpregnant Women: Results From 2 Phase 2 Trials.J Infect Dis. 2018 Apr 23;217(10):1616-1625. doi: 10.1093/infdis/jiy065. J Infect Dis. 2018. PMID: 29401325 Free PMC article. Clinical Trial.
-
Safety and immunogenicity of a combined Tetanus, Diphtheria, recombinant acellular Pertussis vaccine (TdaP) in healthy Thai adults.Hum Vaccin Immunother. 2017 Jan 2;13(1):136-143. doi: 10.1080/21645515.2016.1234555. Epub 2016 Sep 29. Hum Vaccin Immunother. 2017. PMID: 27686283 Free PMC article. Clinical Trial.
-
Reduced-antigen, combined diphtheria, tetanus and acellular pertussis vaccine, adsorbed (Boostrix®): a review of its properties and use as a single-dose booster immunization.Drugs. 2012 Sep 10;72(13):1765-91. doi: 10.2165/11209630-000000000-00000. Drugs. 2012. PMID: 22931522 Review.
-
Prevention of pertussis, tetanus, and diphtheria among pregnant and postpartum women and their infants recommendations of the Advisory Committee on Immunization Practices (ACIP).MMWR Recomm Rep. 2008 May 30;57(RR-4):1-51. MMWR Recomm Rep. 2008. PMID: 18509304 Review.
Cited by
-
Efficacy, immunogenicity and safety of respiratory syncytial virus prefusion F vaccine: systematic review and meta-analysis.BMC Public Health. 2024 May 6;24(1):1244. doi: 10.1186/s12889-024-18748-8. BMC Public Health. 2024. PMID: 38711074 Free PMC article.
-
Vaccine Development for Human Pneumoviruses.Vaccines (Basel). 2025 May 26;13(6):569. doi: 10.3390/vaccines13060569. Vaccines (Basel). 2025. PMID: 40573900 Free PMC article. Review.
-
Protecting infants against RSV disease: an impact and cost-effectiveness comparison of long-acting monoclonal antibodies and maternal vaccination.Lancet Reg Health Eur. 2024 Jan 8;38:100829. doi: 10.1016/j.lanepe.2023.100829. eCollection 2024 Mar. Lancet Reg Health Eur. 2024. PMID: 38476752 Free PMC article.
-
Prenatal Maternal Immunization for Infant Protection: A Review of the Vaccines Recommended, Infant Immunity and Future Research Directions.Pathogens. 2024 Feb 23;13(3):200. doi: 10.3390/pathogens13030200. Pathogens. 2024. PMID: 38535543 Free PMC article. Review.
-
Current strategies and perspectives for active and passive immunization against Respiratory Syncytial Virus in childhood.J Pediatr (Rio J). 2023 Mar-Apr;99 Suppl 1(Suppl 1):S4-S11. doi: 10.1016/j.jped.2022.10.004. Epub 2022 Nov 17. J Pediatr (Rio J). 2023. PMID: 36402228 Free PMC article. Review.
References
-
- Hall CB, Simoes EA, Anderson LJ.. Clinical and epidemiologic features of respiratory syncytial virus. In: Anderson LJ, Graham BS, eds. Challenges and opportunities for respiratory syncytial virus vaccines. Berlin/Heidelberg, Germany: Springer, 2013.
-
- Rha B, Curns AT, Lively JY, et al. . Respiratory syncytial virus-associated hospitalizations among young children: 2015-2016. Pediatrics 2020; 146:e20193611. - PubMed
-
- Geoghegan S, Erviti A, Caballero MT, et al. . Mortality due to respiratory syncytial virus. Burden and risk factors. Am J Respir Crit Care Med 2017; 195:96–103. - PubMed
-
- Laudanno SL, Sánchez Yanotti CI, Polack FP.. RSV lower respiratory tract illness in infants of low- and middle-income countries. Acta Med Acad 2020; 49:191–7. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

