Mechanoreceptor synapses in the brainstem shape the central representation of touch
- PMID: 34637701
- PMCID: PMC8556359
- DOI: 10.1016/j.cell.2021.09.023
Mechanoreceptor synapses in the brainstem shape the central representation of touch
Abstract
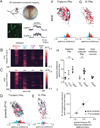
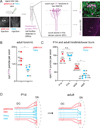
Mammals use glabrous (hairless) skin of their hands and feet to navigate and manipulate their environment. Cortical maps of the body surface across species contain disproportionately large numbers of neurons dedicated to glabrous skin sensation, in part reflecting a higher density of mechanoreceptors that innervate these skin regions. Here, we find that disproportionate representation of glabrous skin emerges over postnatal development at the first synapse between peripheral mechanoreceptors and their central targets in the brainstem. Mechanoreceptor synapses undergo developmental refinement that depends on proximity of their terminals to glabrous skin, such that those innervating glabrous skin make synaptic connections that expand their central representation. In mice incapable of sensing gentle touch, mechanoreceptors innervating glabrous skin still make more powerful synapses in the brainstem. We propose that the skin region a mechanoreceptor innervates controls the developmental refinement of its central synapses to shape the representation of touch in the brain.
Keywords: LTMR; brainstem; disproportionate representation; homunculus; mechanoreceptor; neural activity; peripheral nervous system; piezo2; somatosensation; synaptic expansion; touch.
Copyright © 2021 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures





Similar articles
-
Piezo2 is the major transducer of mechanical forces for touch sensation in mice.Nature. 2014 Dec 4;516(7529):121-5. doi: 10.1038/nature13980. Nature. 2014. PMID: 25471886 Free PMC article.
-
Specialized mechanoreceptor systems in rodent glabrous skin.J Physiol. 2018 Oct;596(20):4995-5016. doi: 10.1113/JP276608. Epub 2018 Sep 15. J Physiol. 2018. PMID: 30132906 Free PMC article.
-
Skin-type-dependent development of murine mechanosensory neurons.Dev Cell. 2023 Oct 23;58(20):2032-2047.e6. doi: 10.1016/j.devcel.2023.07.020. Epub 2023 Aug 21. Dev Cell. 2023. PMID: 37607547 Free PMC article.
-
Touch sense: functional organization and molecular determinants of mechanosensitive receptors.Channels (Austin). 2012 Jul-Aug;6(4):234-45. doi: 10.4161/chan.22213. Channels (Austin). 2012. PMID: 23146937 Free PMC article. Review.
-
Tactile innervation densities across the whole body.J Neurophysiol. 2020 Oct 1;124(4):1229-1240. doi: 10.1152/jn.00313.2020. Epub 2020 Sep 23. J Neurophysiol. 2020. PMID: 32965159 Review.
Cited by
-
Sensory computations in the cuneate nucleus of macaques.Proc Natl Acad Sci U S A. 2021 Dec 7;118(49):e2115772118. doi: 10.1073/pnas.2115772118. Proc Natl Acad Sci U S A. 2021. PMID: 34853173 Free PMC article.
-
Spinal sensory innervation of the intestine.Curr Opin Neurobiol. 2025 Feb;90:102973. doi: 10.1016/j.conb.2025.102973. Epub 2025 Jan 31. Curr Opin Neurobiol. 2025. PMID: 39892315 Free PMC article. Review.
-
Expansion and contraction of resource allocation in sensory bottlenecks.Elife. 2022 Aug 4;11:e70777. doi: 10.7554/eLife.70777. Elife. 2022. PMID: 35924884 Free PMC article.
-
The auditory midbrain mediates tactile vibration sensing.Cell. 2025 Jan 9;188(1):104-120.e18. doi: 10.1016/j.cell.2024.11.014. Epub 2024 Dec 18. Cell. 2025. PMID: 39701100
-
C-LTMRs evoke wet dog shakes via the spinoparabrachial pathway.Science. 2024 Nov 8;386(6722):686-692. doi: 10.1126/science.adq8834. Epub 2024 Nov 7. Science. 2024. PMID: 39509513 Free PMC article.
References
-
- Azzopardi P, and Cowey A (1993). Preferential representation of the fovea in the primary visual cortex. Nature 361, 719–721. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

