An anaplerotic approach to correct the mitochondrial dysfunction in ataxia-telangiectasia (A-T)
- PMID: 34637921
- PMCID: PMC8599162
- DOI: 10.1016/j.molmet.2021.101354
An anaplerotic approach to correct the mitochondrial dysfunction in ataxia-telangiectasia (A-T)
Abstract
Background: ATM, the protein defective in the human genetic disorder, ataxia-telangiectasia (A-T) plays a central role in response to DNA double-strand breaks (DSBs) and in protecting the cell against oxidative stress. We showed that A-T cells are hypersensitive to metabolic stress which can be accounted for by a failure to exhibit efficient endoplasmic reticulum (ER)-mitochondrial signalling and Ca2+ transfer in response to nutrient deprivation resulting in mitochondrial dysfunction. The objective of the current study is to use an anaplerotic approach using the fatty acid, heptanoate (C7), a metabolic product of the triglyceride, triheptanoin to correct the defect in ER-mitochondrial signalling and enhance cell survival of A-T cells in response to metabolic stress.
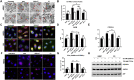
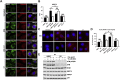
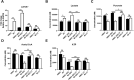
Methods: We treated control cells and A-T cells with the anaplerotic agent, heptanoate to determine their sensitivity to metabolic stress induced by inhibition of glycolysis with 2- deoxyglucose (2DG) using live-cell imaging to monitor cell survival for 72 h using the Incucyte system. We examined ER-mitochondrial signalling in A-T cells exposed to metabolic stress using a suite of techniques including immunofluorescence staining of Grp75, ER-mitochondrial Ca2+ channel, the VAPB-PTPIP51 ER-mitochondrial tether complexes as well as proximity ligation assays between Grp75-IP3R1 and VAPB1-PTPIP51 to establish a functional interaction between ER and mitochondria. Finally, we also performed metabolomic analysis using LC-MS/MS assay to determine altered levels of TCA intermediates A-T cells compared to healthy control cells.
Results: We demonstrate that heptanoate corrects all aspects of the defective ER-mitochondrial signalling observed in A-T cells. Heptanoate enhances ER-mitochondrial contacts; increases the flow of calcium from the ER to the mitochondrion; restores normal mitochondrial function and mitophagy and increases the resistance of ATM-deficient cells and cells from A-T patients to metabolic stress-induced killing. The defect in mitochondrial function in ATM-deficient cells was accompanied by more reliance on aerobic glycolysis as shown by increased lactate dehydrogenase A (LDHA), accumulation of lactate, and reduced levels of both acetyl CoA and ATP which are all restored by heptanoate.
Conclusions: We conclude that heptanoate corrects metabolic stress in A-T cells by restoring ER-mitochondria signalling and mitochondrial function and suggest that the parent compound, triheptanoin, has immense potential as a novel therapeutic agent for patients with A-T.
Keywords: ATM; Ataxia-telangiectasia; Endoplasmic reticulum–mitochondrial interaction; Heptanoate (C7); Mitochondrial dysfunction; Nutrient deprivation.
Copyright © 2021 The Author(s). Published by Elsevier GmbH.. All rights reserved.
Figures




References
-
- Lavin M.F. Ataxia-telangiectasia: from a rare disorder to a paradigm for cell signalling and cancer. Nature Reviews Molecular Cell Biology. 2008;9(10):759–769. - PubMed
-
- McKinnon P.J. ATM and the molecular pathogenesis of ataxia telangiectasia. Annual Review of Pathology: Mechanisms of Disease. 2012;7:303–321. - PubMed
-
- Mirzoeva O.K., Petrini J.H. DNA replication-dependent nuclear dynamics of the Mre11 complex. Molecular Cancer Research. 2003;1(3):207–218. - PubMed
-
- Bakkenist C.J., Kastan M.B. DNA damage activates ATM through intermolecular autophosphorylation and dimer dissociation. Nature. 2003;421(6922):499–506. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

