Lost but Not Least-Novel Insights into Progesterone Receptor Loss in Estrogen Receptor-Positive Breast Cancer
- PMID: 34638241
- PMCID: PMC8507533
- DOI: 10.3390/cancers13194755
Lost but Not Least-Novel Insights into Progesterone Receptor Loss in Estrogen Receptor-Positive Breast Cancer
Abstract
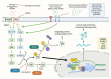
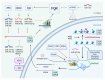
Estrogen receptor α (ERα) and progesterone receptor (PgR) are crucial prognostic and predictive biomarkers that are usually co-expressed in breast cancer (BC). However, 12-24% of BCs present ERα(+)/PgR(-) phenotype at immunohistochemical evaluation. In fact, BC may either show primary PgR(-) status (in chemonaïve tumor sample), lose PgR expression during neoadjuvant treatment, or acquire PgR(-) phenotype in local relapse or metastasis. The loss of PgR expression in ERα(+) breast cancer may signify resistance to endocrine therapy and poorer outcomes. On the other hand, ERα(+)/PgR(-) BCs may have a better response to neoadjuvant chemotherapy than double-positive tumors. Loss of PgR expression may be a result of pre-transcriptional alterations (copy number loss, mutation, epigenetic modifications), decreased transcription of the PGR gene (e.g., by microRNAs), and post-translational modifications (e.g., phosphorylation, sumoylation). Various processes involved in the down-regulation of PgR have distinct consequences on the biology of cancer cells. Occasionally, negative PgR status detected by immunohistochemical analysis is paradoxically associated with enhanced transcriptional activity of PgR that might be inhibited by antiprogestin treatment. Identification of the mechanism of PgR loss in each patient seems challenging, yet it may provide important information on the biology of the tumor and predict its responsiveness to the therapy.
Keywords: breast cancer; estrogen receptor; microRNA; progesterone receptor; treatment.
Conflict of interest statement
The authors declare no conflict of interest. Declaration of interest: M.K., M.P. and W.B. declare no conflict of interest. E.S. discloses relationships with the following entities: Egis, Eli Lilly, Genomic Health, Novartis, Pfizer.
Figures

References
-
- Nordenskjöld A., Fohlin H., Fornander T., Löfdahl B., Skoog L., Stål O. Progesterone receptor positivity is a predictor of long-term benefit from adjuvant tamoxifen treatment of estrogen receptor positive breast cancer. Breast Cancer Res. Treat. 2016;160:313–322. doi: 10.1007/s10549-016-4007-5. - DOI - PMC - PubMed
-
- Braun L., Mietzsch F., Seibold P., Schneeweiss A., Schirmacher P., Chang-Claude J., Peter Sinn H., Aulmann S. Intrinsic breast cancer subtypes defined by estrogen receptor signalling—Prognostic relevance of progesterone receptor loss. Mod. Pathol. 2013;26:1161–1171. doi: 10.1038/modpathol.2013.60. - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

