Fibroblasts Influence Metastatic Melanoma Cell Sensitivity to Combined BRAF and MEK Inhibition
- PMID: 34638245
- PMCID: PMC8507536
- DOI: 10.3390/cancers13194761
Fibroblasts Influence Metastatic Melanoma Cell Sensitivity to Combined BRAF and MEK Inhibition
Abstract
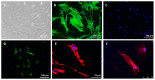
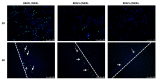
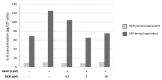
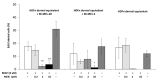
The sensitivity of melanoma cells to targeted therapy compounds depends on the tumor microenvironment. Three-dimensional (3D) in vitro coculture systems better reflect the native structural architecture of tissues and are ideal for investigating cellular interactions modulating cell sensitivity to drugs. Metastatic melanoma (MM) cells (SK-MEL-28 BRAF V600E mutant and SK-MEL-2 BRAF wt) were cultured as a monolayer (2D) or cocultured on 3D dermal equivalents (with fibroblasts) and treated with a BRAFi (vemurafenib) combined with a MEK inhibitor (MEKi, cobimetinib). The drug combination efficiently inhibited 2D and 3D MM cell proliferation and survival regardless of their BRAF status. Two-dimensional and three-dimensional cancer-associated fibroblasts (CAFs), isolated from a cutaneous MM biopsy, were also sensitive to the targeted therapy. Conditioned media obtained from healthy dermal fibroblasts or CAFs modulated the MM cell's response differently to the treatment: while supernatants from healthy fibroblasts potentialized the efficiency of drugs on MM, those from CAFs tended to increase cell survival. Our data indicate that the secretory profiles of fibroblasts influence MM sensitivity to the combined vemurafenib and cobimetinib treatment and highlight the need for 3D in vitro cocultures representing the complex crosstalk between melanoma and CAFs during preclinical studies of drugs.
Keywords: 3D human melanoma model; BRAFi + MEKi efficiency; cancer-associated fibroblasts; targeted therapy; tissue engineering.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

