Phase I/II Study of LDE225 in Combination with Gemcitabine and Nab-Paclitaxel in Patients with Metastatic Pancreatic Cancer
- PMID: 34638351
- PMCID: PMC8507646
- DOI: 10.3390/cancers13194869
Phase I/II Study of LDE225 in Combination with Gemcitabine and Nab-Paclitaxel in Patients with Metastatic Pancreatic Cancer
Abstract
Background: Desmoplasia is a central feature of the tumor microenvironment in pancreatic ductal adenocarcinoma (PDAC). LDE225 is a pharmacological Hedgehog signaling pathway inhibitor and is thought to specifically target tumor stroma. We investigated the combined use of LDE225 and chemotherapy to treat PDAC patients.
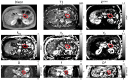
Methods: This was a multi-center, phase I/II study for patients with metastatic PDAC establishing the maximum tolerated dose of LDE225 co-administered with gemcitabine and nab-paclitaxel (phase I) and evaluating the efficacy and safety of the treatment combination after prior FOLFIRINOX treatment (phase II). Tumor microenvironment assessment was performed with quantitative MRI using intra-voxel incoherent motion diffusion weighted MRI (IVIM-DWI) and dynamic contrast-enhanced (DCE) MRI.
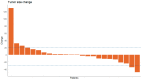
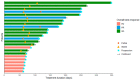
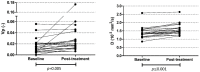
Results: The MTD of LDE225 was 200 mg once daily co-administered with gemcitabine 1000 mg/m2 and nab-paclitaxel 125 mg/m2. In phase II, six therapy-related grade 4 adverse events (AE) and three grade 5 were observed. In 24 patients, the target lesion response was evaluable. Three patients had partial response (13%), 14 patients showed stable disease (58%), and 7 patients had progressive disease (29%). Median overall survival (OS) was 6 months (IQR 3.9-8.1). Blood plasma fraction (DCE) and diffusion coefficient (IVIM-DWI) significantly increased during treatment. Baseline perfusion fraction could predict OS (>222 days) with 80% sensitivity and 85% specificity.
Conclusion: LDE225 in combination with gemcitabine and nab-paclitaxel was well-tolerated in patients with metastatic PDAC and has promising efficacy after prior treatment with FOLFIRINOX. Quantitative MRI suggested that LDE225 causes increased tumor diffusion and works particularly well in patients with poor baseline tumor perfusion.
Keywords: Hedgehog signaling pathway inhibitor; LDE225; metastatic pancreatic ductal adenocarcinoma; pancreatic neoplasms; quantitative MRI.
Conflict of interest statement
J.W.W. has served as a consultant for Shire, Servier and Celgene and reports grants from Servier, Halozyne, Novartis, Celgene, Astra Zeneca, Pfizer, Roche, Amgen and Merck. HWMvL reports a consult/advisory role for BMS, Celgene, Lilly, Merck, and Nordic, and Servier and has received unrestricted research funding from Bayer, BMS, Celgene, Lilly, Merck Serono, MSD, Nordic, Philips, Roche and Servier. The other authors declare that they have no conflict of interest.
Figures

References
-
- Zhang X.-W., Ma Y.-X., Sun Y., Cao Y.-B., Li Q., Xu C.-A. Gemcitabine in combination with a second cytotoxic agent in the first-line treatment of locally advanced or metastatic pancreatic cancer: A systematic review and meta-analysis. Target. Oncol. 2017;12:309–321. doi: 10.1007/s11523-017-0486-5. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

