Targetable Pathways in Advanced Bladder Cancer: FGFR Signaling
- PMID: 34638374
- PMCID: PMC8507635
- DOI: 10.3390/cancers13194891
Targetable Pathways in Advanced Bladder Cancer: FGFR Signaling
Abstract
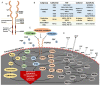
Bladder cancer is the 10th most commonly diagnosed cancer in the world, accounting for around 573,000 new cases and 213,000 deaths in 2020. The current standard treatment for locally advanced bladder cancer is neoadjuvant cisplatin (NAC)-based chemotherapy followed by cystectomy. The significant progress being made in the genomic and molecular understandings of bladder cancer has uncovered the genetic alterations and signaling pathways that drive bladder cancer progression. These developments have led to a dramatic increase in the evaluation of molecular agents targeting at these alterations. One example is Erdafitinib, a first-in-class FGFR inhibitor being approved as second-line treatment for locally advanced or metastatic urothelial carcinoma with FGFR mutations. Immunotherapy has also been approved as second-line treatment for advanced and metastatic bladder cancer. Preclinical studies suggest targeted therapy combined with immunotherapy has the potential to markedly improve patient outcome. Given the prevalence of FGFR alternations in bladder cancer, here we review recent preclinical and clinical studies on FGFR inhibitors and analyze possible drug resistance mechanisms to these agents. We also discuss FGFR inhibitors in combination with other therapies and its potential to improve outcome.
Keywords: DNA; bladder cancer; erdafitinib; fibroblast growth factor receptor; kinase inhibitors; mutation; targeted therapy.
Conflict of interest statement
J.E.D. receives compensation from Invitae Corporation. All other authors have no personal circumstances or interest that may be perceived as inappropriately influencing the representation or interpretation of review article.
Figures
References
-
- Westergren D.-O., Gårdmark T., Lindhagen L., Chau A., Malmström P.-U. A Nationwide, Population Based Analysis of Patients with Organ Confined, Muscle Invasive Bladder Cancer Not Receiving Curative Intent Therapy in Sweden from 1997 to 2014. J. Urol. 2019;202:905–912. doi: 10.1097/JU.0000000000000350. - DOI - PubMed
-
- Grossman H.B., Natale R.B., Tangen C.M., Speights V., Vogelzang N.J., Trump D.L., White R.W.D., Sarosdy M.F., Wood D.P., Raghavan D., et al. Neoadjuvant Chemotherapy plus Cystectomy Compared with Cystectomy Alone for Locally Advanced Bladder Cancer. N. Engl. J. Med. 2003;349:859–866. doi: 10.1056/NEJMoa022148. - DOI - PubMed
-
- Fairey A.S., Daneshmand S., Quinn D., Dorff T., Dorin R., Lieskovsky G., Schuckman A., Cai J., Miranda G., Skinner E.C. Neoadjuvant chemotherapy with gemcitabine/cisplatin vs. methotrexate/vinblastine/doxorubicin/cisplatin for muscle-invasive urothelial carcinoma of the bladder: A retrospective analysis from the University of Southern California. Urol. Oncol. Semin. Orig. Investig. 2012;31:1737–1743. doi: 10.1016/j.urolonc.2012.07.005. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

