Biologically Targeted Radiation Therapy: Incorporating Patient-Specific Hypoxia Data Derived from Quantitative Magnetic Resonance Imaging
- PMID: 34638382
- PMCID: PMC8507789
- DOI: 10.3390/cancers13194897
Biologically Targeted Radiation Therapy: Incorporating Patient-Specific Hypoxia Data Derived from Quantitative Magnetic Resonance Imaging
Abstract
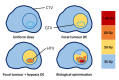
Purpose: Hypoxia has been linked to radioresistance. Strategies to safely dose escalate dominant intraprostatic lesions have shown promising results, but further dose escalation to overcome the effects of hypoxia require a novel approach to constrain the dose in normal tissue.to safe levels. In this study, we demonstrate a biologically targeted radiotherapy (BiRT) approach that can utilise multiparametric magnetic resonance imaging (mpMRI) to target hypoxia for favourable treatment outcomes.
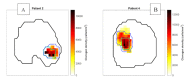
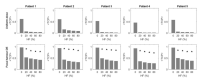
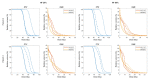
Methods: mpMRI-derived tumour biology maps, developed via a radiogenomics study, were used to generate individualised, hypoxia-targeting prostate IMRT plans using an ultra- hypofractionation schedule. The spatial distribution of mpMRI textural features associated with hypoxia-related genetic profiles was used as a surrogate of tumour hypoxia. The effectiveness of the proposed approach was assessed by quantifying the potential benefit of a general focal boost approach on tumour control probability, and also by comparing the dose to organs at risk (OARs) with hypoxia-guided focal dose escalation (DE) plans generated for five patients.
Results: Applying an appropriately guided focal boost can greatly mitigate the impact of hypoxia. Statistically significant reductions in rectal and bladder dose were observed for hypoxia-targeting, biologically optimised plans compared to isoeffective focal DE plans.
Conclusion: Results of this study suggest the use of mpMRI for voxel-level targeting of hypoxia, along with biological optimisation, can provide a mechanism for guiding focal DE that is considerably more efficient than application of a general, dose-based optimisation, focal boost.
Keywords: hypoxia; multiparametric MRI; prostate cancer; radiogenomics; tumour control probability.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results. SW has received consultancy fees and/or travel support paid to his institution from Astellas, Janssen, Bayer, Amgen and Noxopharm. SW has received scientific grant support from Bayer and Astella pharmaceuticals for clinical trials administered through the Australia and New Zealand Urogenital and Prostate (ANZUP) clinical trials group. SW also has research supported by Bristol-Myers Squibb, Prostate Cancer Foundation (USA), Prostate Cancer Foundation Australia, the PeterMac Foundation, Cancer Council Victoria and Victoria Cancer Agency. None of these relate to the current work. AH has a non-financial research agreement with Siemens Healthineers which does not relate to the current work.
Figures


References
-
- Milosevic M., Warde P., Ménard C., Chung P., Toi A., Ishkanian A., McLean M., Pintilie M., Sykes J., Gospodarowicz M., et al. Tumor Hypoxia Predicts Biochemical Failure following Radiotherapy for Clinically Localized Prostate Cancer. Clin. Cancer Res. 2012;18:2108–2114. doi: 10.1158/1078-0432.CCR-11-2711. - DOI - PubMed
-
- Kerkmeijer L.G.W., Groen V.H., Pos F.J., Haustermans K., Monninkhof E.M., Smeenk R.J., Kunze-Busch M., de Boer J.C.J., Zijp J.V.D.V.V., van Vulpen M., et al. Focal Boost to the Intraprostatic Tumor in External Beam Radiotherapy for Patients With Localized Prostate Cancer: Results From the FLAME Randomized Phase III Trial. J. Clin. Oncol. 2021;39:787–796. doi: 10.1200/JCO.20.02873. - DOI - PubMed
-
- Draulans C., van der Heide U.A., Haustermans K., Pos F.J., Zyp J.V.D.V.V., De Boer H., Groen V.H., Monninkhof E.M., Smeenk R.J., Kunze-Busch M., et al. Primary endpoint analysis of the multicentre phase II hypo-FLAME trial for intermediate and high risk prostate cancer. Radiother. Oncol. 2020;147:92–98. doi: 10.1016/j.radonc.2020.03.015. - DOI - PubMed
-
- Duchesne G.M., Peters L.J. What is the alpha/beta ratio for prostate cancer? Rationale for hypofractionated high-dose-rate brachytherapy. Int. J. Radiat Oncol. Biol. Phys. 1999;44:747–748. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

