Interobserver Agreement of PD-L1/SP142 Immunohistochemistry and Tumor-Infiltrating Lymphocytes (TILs) in Distant Metastases of Triple-Negative Breast Cancer: A Proof-of-Concept Study. A Report on Behalf of the International Immuno-Oncology Biomarker Working Group
- PMID: 34638394
- PMCID: PMC8507620
- DOI: 10.3390/cancers13194910
Interobserver Agreement of PD-L1/SP142 Immunohistochemistry and Tumor-Infiltrating Lymphocytes (TILs) in Distant Metastases of Triple-Negative Breast Cancer: A Proof-of-Concept Study. A Report on Behalf of the International Immuno-Oncology Biomarker Working Group
Abstract
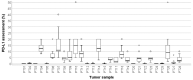
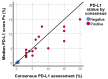
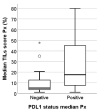
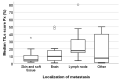
Patients with advanced triple-negative breast cancer (TNBC) benefit from treatment with atezolizumab, provided that the tumor contains ≥1% of PD-L1/SP142-positive immune cells. Numbers of tumor-infiltrating lymphocytes (TILs) vary strongly according to the anatomic localization of TNBC metastases. We investigated inter-pathologist agreement in the assessment of PD-L1/SP142 immunohistochemistry and TILs. Ten pathologists evaluated PD-L1/SP142 expression in a proficiency test comprising 28 primary TNBCs, as well as PD-L1/SP142 expression and levels of TILs in 49 distant TNBC metastases with various localizations. Interobserver agreement for PD-L1 status (positive vs. negative) was high in the proficiency test: the corresponding scores as percentages showed good agreement with the consensus diagnosis. In TNBC metastases, there was substantial variability in PD-L1 status at the individual patient level. For one in five patients, the chance of treatment was essentially random, with half of the pathologists designating them as positive and half negative. Assessment of PD-L1/SP142 and TILs as percentages in TNBC metastases showed poor and moderate agreement, respectively. Additional training for metastatic TNBC is required to enhance interobserver agreement. Such training, focusing on metastatic specimens, seems worthwhile, since the same pathologists obtained high percentages of concordance (ranging from 93% to 100%) on the PD-L1 status of primary TNBCs.
Keywords: PD-L1; SP142; TILs; TNBC; atezolizumab; distant metastasis; immune cells; interobserver variability; triple-negative breast cancer; tumor-infiltrating lymphocytes.
Conflict of interest statement
J.M.S.B. received research funding from Thermo Fisher Scientific, Genoptix, Agendia, Nanostring Technologies Inc., Stratifyer GmbH and Biotheranostics Inc., as well as honoraria from NanoString Technologies Inc., Oncology Education, Biotheranostics Inc., and MedcomXchange Communications Inc. M.K. (Marleen Kok) received institutional funding from AZ, BMS, and Roche. M.K. (Marleen Kok) is an advisory board member of BMS, Daiichi, MSD, and Roche. C.H.M.v.D. received funding for this study from Roche. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results. The other authors declare no conflict of interest.
Figures

References
-
- Dodson A., Parry S., Ibrahim M., Bartlett J.M.S., Pinder S., Dowsett M., Miller K. Breast cancer biomarkers in clinical testing: Analysis of a UK national external quality assessment scheme for immunocytochemistry and in situ hybridisation database containing results from 199,300 patients. J. Pathol. Clin. Res. 2018;4:262–273. doi: 10.1002/cjp2.112. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

