Remodeling the ECM: Implications for Metastasis and Tumor Dormancy
- PMID: 34638400
- PMCID: PMC8507703
- DOI: 10.3390/cancers13194916
Remodeling the ECM: Implications for Metastasis and Tumor Dormancy
Abstract
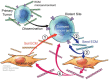
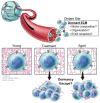
While most primary tumors can be effectively treated, therapeutics fail to efficiently eliminate metastases. Metastases arise from cancer cells that leave the primary tumor and seed distant sites. Recent studies have shown that cancer cells disseminate early during tumor progression and can remain dormant for years before they resume growth. In these metastatic organs, cancer cells reside in microenvironments where they interact with other cells, but also with the extracellular matrix (ECM). The ECM was long considered to be an inert, non-cellular component of tissues, providing their architecture. However, in recent years, a growing body of evidence has shown that the ECM is a key driver of cancer progression, and it can exert effects on tumor cells, regulating their metastatic fate. ECM remodeling and degradation is required for the early steps of the metastatic cascade: invasion, tumor intravasation, and extravasation. Similarly, ECM molecules have been shown to be important for metastatic outgrowth. However, the role of ECM molecules on tumor dormancy and their contribution to the dormancy-supportive niches is not well understood. In this perspective article, we will summarize the current knowledge of ECM and its role in tumor metastasis and dormancy. We will discuss how a better understanding of the individual components of the ECM niche and their roles mediating the dormant state of disseminated tumor cells (DTCs) will advance the development of new therapies to target dormant cells and prevent metastasis outgrowth.
Keywords: ECM; cancer dormancy; metastasis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Grants and funding
LinkOut - more resources
Full Text Sources

