Light Therapy for Cancer-Related Fatigue in (Non-)Hodgkin Lymphoma Survivors: Results of a Randomized Controlled Trial
- PMID: 34638428
- PMCID: PMC8508131
- DOI: 10.3390/cancers13194948
Light Therapy for Cancer-Related Fatigue in (Non-)Hodgkin Lymphoma Survivors: Results of a Randomized Controlled Trial
Abstract
Purpose: To evaluate the short- and long-term effects of light therapy on fatigue (primary outcome) and sleep quality, depression, anxiety, quality of life, and circadian rhythms (secondary outcomes) in survivors of (non-)Hodgkin lymphoma presenting with chronic cancer-related fatigue.
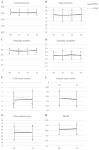
Methods: We randomly assigned 166 survivors (mean survival 13 years) to a bright white light intervention (BWL) or dim white light comparison (DWL) group. Measurements were completed at baseline (T0), post-intervention (T1), at three (T2), and nine (T3) months follow-up. A mixed-effect modeling approach was used to compare linear and non-linear effects of time between groups.
Results: There were no significant differences between BWL and DWL in the reduction in fatigue over time. Both BWL and DWL significantly (p < 0.001) improved fatigue levels during the intervention followed by a slight reduction in this effect during follow-up (EST0-T1 = -0.71; EST1-T3 = 0.15). Similar results were found for depression, sleep quality, and some aspects of quality of life. Light therapy had no effect on circadian rhythms.
Conclusions: BWL was not superior in reducing fatigue compared to DWL in HL and DLBCL survivors. Remarkably, the total sample showed clinically relevant and persistent improvements on fatigue not commonly seen in longitudinal observational studies in these survivors.
Keywords: cancer-related fatigue; circadian rhythms; light therapy; randomized controlled trial; sleep.
Conflict of interest statement
M.J.K. has received institutional research funding from Kite/Gilead and Novartis outsides the submitted work. L.H.B. has received a travel grant from Servier. S.A.-I. is an advisory board member for Eisai and Merck. All remaining authors have declared no conflicts of interest.
Figures


References
-
- Oerlemans S., Mols F., Issa D.E., Pruijt J., Peters W.G., Lybeert M., Zijlstra W., Coebergh J.W.W., van de Poll-Franse L.V. A high level of fatigue among long-term survivors of non-Hodgkin’s lymphoma: Results from the longitudinal population-based PROFILES registry in the south of the Netherlands. Haematologica. 2013;98:479–486. doi: 10.3324/haematol.2012.064907. - DOI - PMC - PubMed
-
- Busson R., van der Kaaij M., Mounier N., Aleman B.M., Thiéblemont C., Stamatoullas A., Ribrag V., Tilly H., Haioun C., Casasnovas R.-O. Fatigue level changes with time in long-term Hodgkin and non-Hodgkin lymphoma survivors: A joint EORTC-LYSA cross-sectional study. Health Qual. Life Outcomes. 2019;17:1–13. doi: 10.1186/s12955-019-1186-x. - DOI - PMC - PubMed
-
- Mounier N., Anthony S., Busson R., Thieblemont C., Ribrag V., Tilly H., Haioun C., Casasnovas R.O., Morschhauser F., Feugier P. Long-term fatigue in survivors of non-Hodgkin lymphoma: The Lymphoma Study Association SIMONAL cross-sectional study. Cancer. 2019;125:2291–2299. doi: 10.1002/cncr.32040. - DOI - PubMed
-
- Bower J.E., Bak K., Berger A., Breitbart W., Escalante C.P., Ganz P.A., Schnipper H.H., Lacchetti C., Ligibel J.A., Lyman G.H. Screening, assessment, and management of fatigue in adult survivors of cancer: An American Society of Clinical oncology clinical practice guideline adaptation. J. Clin. Oncol. 2014;32:1840–1850. doi: 10.1200/JCO.2013.53.4495. - DOI - PMC - PubMed

