Novel Simple Conjugation Chemistries for Decoration of GMMA with Heterologous Antigens
- PMID: 34638530
- PMCID: PMC8508390
- DOI: 10.3390/ijms221910180
Novel Simple Conjugation Chemistries for Decoration of GMMA with Heterologous Antigens
Abstract
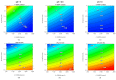
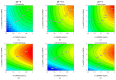
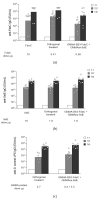
Outer Membrane Vesicles (OMV) constitute a promising platform for the development of efficient vaccines. OMV can be decorated with heterologous antigens (proteins or polysaccharides), becoming attractive novel carriers for the development of multicomponent vaccines. Chemical conjugation represents a tool for linking antigens, also from phylogenetically distant pathogens, to OMV. Here we develop two simple and widely applicable conjugation chemistries targeting proteins or lipopolysaccharides on the surface of Generalized Modules for Membrane Antigens (GMMA), OMV spontaneously released from Gram-negative bacteria mutated to increase vesicle yield and reduce potential reactogenicity. A Design of Experiment approach was used to identify optimal conditions for GMMA activation before conjugation, resulting in consistent processes and ensuring conjugation efficiency. Conjugates produced by both chemistries induced strong humoral response against the heterologous antigen and GMMA. Additionally, the use of the two orthogonal chemistries allowed to control the linkage of two different antigens on the same GMMA particle. This work supports the further advancement of this novel platform with great potential for the design of effective vaccines.
Keywords: GMMA; OMV; carrier protein; conjugation chemistry; glycoconjugate; vaccine.
Conflict of interest statement
This work was undertaken at the request of and sponsored by GlaxoSmithKline Biologicals SA. All authors were employees of the GSK group of Companies when the study was performed. R.D.B., R.A., A.S. and F.M. are listed as inventors on patents related to this work owned by the GSK group of companies.
Figures








References
-
- Valguarnera E., Feldman M.F. Glycoengineered Outer Membrane Vesicles as a Platform for Vaccine Development. Methods Enzymol. 2017;597:285–310. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

