Super-Resolution Imaging of the A- and B-Type Lamin Networks: A Comparative Study of Different Fluorescence Labeling Procedures
- PMID: 34638534
- PMCID: PMC8508656
- DOI: 10.3390/ijms221910194
Super-Resolution Imaging of the A- and B-Type Lamin Networks: A Comparative Study of Different Fluorescence Labeling Procedures
Abstract
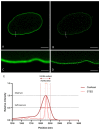
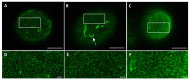
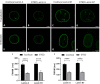
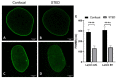
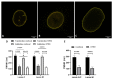
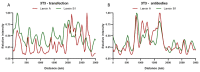
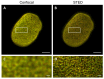
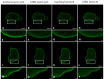
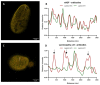
A- and B-type lamins are type V intermediate filament proteins. Mutations in the genes encoding these lamins cause rare diseases, collectively called laminopathies. A fraction of the cells obtained from laminopathy patients show aberrations in the localization of each lamin subtype, which may represent only the minority of the lamina disorganization. To get a better insight into more delicate and more abundant lamina abnormalities, the lamin network can be studied using super-resolution microscopy. We compared confocal scanning laser microscopy and stimulated emission depletion (STED) microscopy in combination with different fluorescence labeling approaches for the study of the lamin network. We demonstrate the suitability of an immunofluorescence staining approach when using STED microscopy, by determining the lamin layer thickness and the degree of lamin A and B1 colocalization as detected in fixed fibroblasts (co-)stained with lamin antibodies or (co-)transfected with EGFP/YFP lamin constructs. This revealed that immunofluorescence staining of cells does not lead to consequent changes in the detected lamin layer thickness, nor does it influence the degree of colocalization of lamin A and B1, when compared to the transfection approach. Studying laminopathy patient dermal fibroblasts (LMNA c.1130G>T (p.(Arg377Leu)) variant) confirmed the suitability of immunofluorescence protocols in STED microscopy, which circumvents the need for less convenient transfection steps. Furthermore, we found a significant decrease in lamin A/C and B1 colocalization in these patient fibroblasts, compared to normal human dermal fibroblasts. We conclude that super-resolution light microscopy combined with immunofluorescence protocols provides a potential tool to detect structural lamina differences between normal and laminopathy patient fibroblasts.
Keywords: STED microscopy; antibodies; colocalization; confocal scanning laser microscopy; lamin layer thickness; laminopathy; nuclear lamins; resolution; transfection.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

