Difluoromethylornithine Induces Apoptosis through Regulation of AP-1 Signaling via JNK Phosphorylation in Epithelial Ovarian Cancer
- PMID: 34638596
- PMCID: PMC8508876
- DOI: 10.3390/ijms221910255
Difluoromethylornithine Induces Apoptosis through Regulation of AP-1 Signaling via JNK Phosphorylation in Epithelial Ovarian Cancer
Abstract
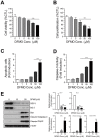
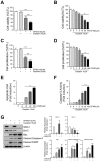
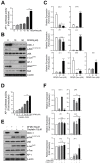
Difluoromethylornithine (DFMO), an irreversible inhibitor of ornithine decarboxylase (ODC), has promising activity against various cancers and a tolerable safety profile for long-term use as a chemopreventive agent. However, the anti-tumor effects of DFMO in ovarian cancer cells have not been entirely understood. Our study aimed to identify the effects and mechanism of DFMO in epithelial ovarian cancer cells using SKOV-3 cells. Treatment with DFMO resulted in a significantly reduced cell viability in a time- and dose-dependent manner. DFMO treatment inhibited the activity and downregulated the expression of ODC in ovarian cancer cells. The reduction in cell viability was reversed using polyamines, suggesting that polyamine depletion plays an important role in the anti-tumor activity of DFMO. Additionally, significant changes in Bcl-2, Bcl-xL, Bax protein levels, activation of caspase-3, and cleavage of poly (ADP-ribose) polymerase were observed, indicating the apoptotic effects of DFMO. We also found that the effect of DFMO was mediated by AP-1 through the activation of upstream JNK via phosphorylation. Moreover, DFMO enhanced the effect of cisplatin, thus showing a possibility of a synergistic effect in treatment. In conclusion, treatment with DFMO alone, or in combination with cisplatin, could be a promising treatment for ovarian cancer.
Keywords: AP-1; DFMO; JNK; apoptosis; ovarian cancer; polyamines.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Tothill R., Tinker A.V., George J., Brown R., Fox S., Lade S., Johnson D.S., Trivett M.K., Etemadmoghadam D., Locandro B., et al. Novel Molecular Subtypes of Serous and Endometrioid Ovarian Cancer Linked to Clinical Outcome. Clin. Cancer Res. 2008;14:5198–5208. doi: 10.1158/1078-0432.CCR-08-0196. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

