Platelet Inhibition Prevents NLRP3 Inflammasome Activation and Sepsis-Induced Kidney Injury
- PMID: 34638670
- PMCID: PMC8508664
- DOI: 10.3390/ijms221910330
Platelet Inhibition Prevents NLRP3 Inflammasome Activation and Sepsis-Induced Kidney Injury
Abstract
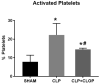
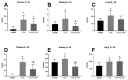
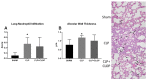
Platelets, cellular mediators of thrombosis, are activated during sepsis and are increasingly recognized as mediators of the immune response. Platelet activation is significantly increased in sepsis patients compared to ICU control patients. Despite this correlation, the role of activated platelets in contributing to sepsis pathophysiology remains unclear. We previously demonstrated NOD-like receptor protein 3 inflammasome (NLRP3) inflammasome activation in sepsis-induced platelets from cecal-ligation puncture (CLP) rats. Activated platelets were associated with increased pulmonary edema and glomerular injury in CLP vs. SHAM controls. In this study, we investigated whether inhibition of platelet activation would attenuate NLRP3 activation and renal and pulmonary injury in response to CLP. CLP was performed in male and female Sprague Dawley (SD) rats (n = 10/group) to induce abdominal sepsis and SHAM rats served as controls. A subset of CLP animals was treated with Clopidogrel (10 mg/kg/day, CLP + CLOP) to inhibit platelet activation. At 72 h post-CLP, platelet activation and NLRP3 inflammasome assembly were evaluated, IL-1β and IL-18 were measured in plasma, and tissues, renal and pulmonary pathology, and renal function were assessed. Activated platelets were 7.8 ± 3.6% in Sham, 22 ± 6% in CLP and significantly decreased to 14.5 ± 0.6% in CLP + CLOP (n = 8-10/group, p < 0.05). NLRP3 inflammasome assembly was inhibited in platelets of CLP + CLOP animals vs. CLP. Significant increases in plasma and kidney IL-1β and IL-18 in response to CLP were decreased with Clopidogrel treatment. Renal injury, but not lung histology or renal function was improved in CLP + CLOP vs. CLP. These data provide evidence that activated platelets may contribute to sepsis-induced renal injury, possibly via NLRP3 activation in platelets. Platelets may be a therapeutic target to decrease renal injury in septic patients.
Keywords: Clopidogrel; NLRP3; endothelial activation; inflammation; multi-organ injury; platelets; sepsis.
Conflict of interest statement
The authors express no conflict of interest.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

