B Cell Lymphoma 2: A Potential Therapeutic Target for Cancer Therapy
- PMID: 34638779
- PMCID: PMC8509036
- DOI: 10.3390/ijms221910442
B Cell Lymphoma 2: A Potential Therapeutic Target for Cancer Therapy
Abstract
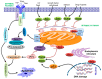
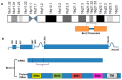
Defects in the apoptosis mechanism stimulate cancer cell growth and survival. B cell lymphoma 2 (Bcl-2) is an anti-apoptotic molecule that plays a central role in apoptosis. Bcl-2 is the founding constituent of the Bcl-2 protein family of apoptosis controllers, the primary apoptosis regulators linked with cancer. Bcl-2 has been identified as being over-expressed in several cancers. Bcl-2 is induced by protein kinases and several signaling molecules which stimulate cancer development. Identifying the important function played by Bcl-2 in cancer progression and development, and treatment made it a target related to therapy for multiple cancers. Among the various strategies that have been proposed to block Bcl-2, BH3-mimetics have appeared as a novel group of compounds thanks to their favorable effects on many cancers within several clinical settings. Because of the fundamental function of Bcl-2 in the regulation of apoptosis, the Bcl-2 protein is a potent target for the development of novel anti-tumor treatments. Bcl-2 inhibitors have been used against several cancers and provide a pre-clinical platform for testing novel therapeutic drugs. Clinical trials of multiple investigational agents targeting Bcl-2 are ongoing. This review discusses the role of Bcl-2 in cancer development; it could be exploited as a potential target for developing novel therapeutic strategies to combat various types of cancers. We further highlight the therapeutic activity of Bcl-2 inhibitors and their implications for the therapeutic management of cancer.
Keywords: B cell lymphoma 2; apoptosis; cancers; clinical trials; inhibitors; targeted therapy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Kiyoshima T., Yoshida H., Wada H., Nagata K., Fujiwara H., Kihara M., Hasegawa K., Someya H., Sakai H. Chemoresistance to concanamycin a1 in human oral squamous cell carcinoma is attenuated by an hdac inhibitor partly via suppression of bcl-2 expression. PLoS ONE. 2013;8:e80998. doi: 10.1371/journal.pone.0080998. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

