TNAP: A New Multitask Enzyme in Energy Metabolism
- PMID: 34638808
- PMCID: PMC8509042
- DOI: 10.3390/ijms221910470
TNAP: A New Multitask Enzyme in Energy Metabolism
Abstract
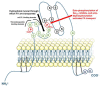
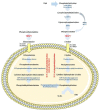
Tissue-nonspecific alkaline phosphatase (TNAP) is mainly known for its necessary role in skeletal and dental mineralization, which relies on the hydrolysis of the mineralization inhibitor inorganic pyrophosphate (PPi). Mutations in the gene encoding TNAP leading to severe hypophosphatasia result in strongly reduced mineralization and perinatal death. Fortunately, the relatively recent development of a recombinant TNAP with a bone anchor has allowed to correct the bone defects and prolong the life of affected babies and children. Researches on TNAP must however not be slowed down, because accumulating evidence indicates that TNAP activation in individuals with metabolic syndrome (MetS) is associated with enhanced cardiovascular mortality, presumably in relation with cardiovascular calcification. On the other hand, TNAP appears to be necessary to prevent the development of steatohepatitis in mice, suggesting that TNAP plays protective roles. The aim of the present review is to highlight the known or suspected functions of TNAP in energy metabolism that may be associated with the development of MetS. The location of TNAP in liver and its function in bile excretion, lipopolysaccharide (LPS) detoxification and fatty acid transport will be presented. The expression and function of TNAP in adipocyte differentiation and thermogenesis will also be discussed. Given that TNAP is a tissue- and substrate-nonspecific phosphatase, we believe that it exerts several crucial pathophysiological functions that are just beginning to be discovered.
Keywords: CD36; TNAP; adipocyte; bile; lipopolysaccharide; liver; metabolic syndrome; phosphocreatine; steatosis.
Conflict of interest statement
The author declares no conflict of interest.
Figures



References
-
- Buchet R., Millán J.L., Magne D. Multisystemic functions of alkaline phosphatases. Methods Mol. Biol. 2013;1053:27–51. - PubMed
-
- Magnusson P., Degerblad M., Sääf M., Larsson L., Thorén M. Different responses of bone alkaline phosphatase isoforms during recombinant insulin-like growth factor-I (IGF-I) and during growth hormone therapy in adults with growth hormone deficiency. J. Bone Miner. Res. 1997;12:210–220. doi: 10.1359/jbmr.1997.12.2.210. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

