Untargeted Metabolic Profiling of Extracellular Vesicles of SARS-CoV-2-Infected Patients Shows Presence of Potent Anti-Inflammatory Metabolites
- PMID: 34638812
- PMCID: PMC8509011
- DOI: 10.3390/ijms221910467
Untargeted Metabolic Profiling of Extracellular Vesicles of SARS-CoV-2-Infected Patients Shows Presence of Potent Anti-Inflammatory Metabolites
Abstract
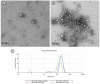
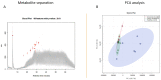
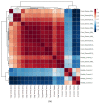
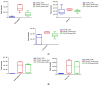
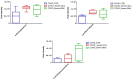
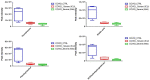
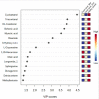
Extracellular vesicles (EVs) carry important biomolecules, including metabolites, and contribute to the spread and pathogenesis of some viruses. However, to date, limited data are available on EV metabolite content that might play a crucial role during infection with the SARS-CoV-2 virus. Therefore, this study aimed to perform untargeted metabolomics to identify key metabolites and associated pathways that are present in EVs, isolated from the serum of COVID-19 patients. The results showed the presence of antivirals and antibiotics such as Foscarnet, Indinavir, and lymecycline in EVs from patients treated with these drugs. Moreover, increased levels of anti-inflammatory metabolites such as LysoPS, 7-α,25-Dihydroxycholesterol, and 15-d-PGJ2 were detected in EVs from COVID-19 patients when compared with controls. Further, we found decreased levels of metabolites associated with coagulation, such as thromboxane and elaidic acid, in EVs from COVID-19 patients. These findings suggest that EVs not only carry active drug molecules but also anti-inflammatory metabolites, clearly suggesting that exosomes might play a crucial role in negotiating with heightened inflammation during COVID-19 infection. These preliminary results could also pave the way for the identification of novel metabolites that might act as critical regulators of inflammatory pathways during viral infections.
Keywords: 15-d-PGJ2; 7-α,25-Dihydroxycholesterol; COVID-19; extracellular vesicles; metabolomics.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
-
- Prompetchara E., Ketloy C., Palaga T. Immune responses in COVID-19 and potential vaccines: Lessons learned from sars and mers epidemic. Asian Pac. J. Allergy Immunol. 2020;38:1–9. - PubMed
-
- Hoffmann M., Kleine-Weber H., Schroeder S., Kruger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.H., Nitsche A., et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280.e278. doi: 10.1016/j.cell.2020.02.052. - DOI - PMC - PubMed
-
- Ma Y., Huang Y., Wang T., Xiang A.P., Huang W. ACE2 shedding and furin abundance in target organs may influence the efficiency of SARS-CoV-2 entry. Open Bioinform. J. 2021;14:1–12. doi: 10.2174/1875036202114010001. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

