Concepts in Oncolytic Adenovirus Therapy
- PMID: 34638863
- PMCID: PMC8508870
- DOI: 10.3390/ijms221910522
Concepts in Oncolytic Adenovirus Therapy
Abstract
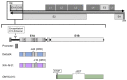
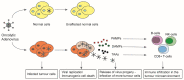
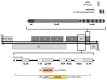
Oncolytic adenovirus therapy is gaining importance as a novel treatment option for the management of various cancers. Different concepts of modification within the adenovirus vector have been identified that define the mode of action against and the interaction with the tumour. Adenoviral vectors allow for genetic manipulations that restrict tumour specificity and also the expression of specific transgenes in order to support the anti-tumour effect. Additionally, replication of the virus and reinfection of neighbouring tumour cells amplify the therapeutic effect. Another important aspect in oncolytic adenovirus therapy is the virus induced cell death which is a process that activates the immune system against the tumour. This review describes which elements in adenovirus vectors have been identified for modification not only to utilize oncolytic adenovirus vectors into conditionally replicating adenoviruses (CRAds) that allow replication specifically in tumour cells but also to confer specific characteristics to these viruses. These advances in development resulted in clinical trials that are summarized based on the conceptual design.
Keywords: CRAd; adenovirus; arming; cancer; immunotherapy; oncolytic virus; tropism; vector design.
Conflict of interest statement
P.S.H. is co-founder of XVir Therapeutics GmbH. All other authors declare no competing interests.
Figures
References
-
- Roelvink P.W., Kovesdi I., Wickham T.J. Comparative analysis of adenovirus fiber-cell interaction: Adenovirus type 2 (Ad2) and Ad9 utilize the same cellular fiber receptor but use different binding strategies for attachment. J. Virol. 1996;70:7614–7621. doi: 10.1128/jvi.70.11.7614-7621.1996. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

