Cyclodextrins Allow the Combination of Incompatible Vancomycin and Ceftazidime into an Ophthalmic Formulation for the Treatment of Bacterial Keratitis
- PMID: 34638878
- PMCID: PMC8508691
- DOI: 10.3390/ijms221910538
Cyclodextrins Allow the Combination of Incompatible Vancomycin and Ceftazidime into an Ophthalmic Formulation for the Treatment of Bacterial Keratitis
Abstract
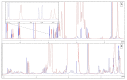
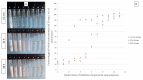
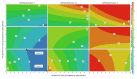
Ceftazidime (CZ) and vancomycin (VA) are two antibiotics used to treat bacterial keratitis. Due to their physical incompatibility (formation of a precipitate), it is not currently possible to associate both molecules in a single container for ophthalmic administration. We firstly characterized the incompatibility then investigated if 2-hydroxypropyl-beta (HPβCD) and 2-hydroxypropyl-gamma cyclodextrins (HPγCD) could prevent this incompatibility. The impact of pH on the precipitation phenomena was investigated by analysing the supernatant solution of the mixture using high performance liquid chromatography. A characterization of the inclusion of CZ with HPγCD using 1H nuclear magnetic resonance (NMR), and VA with HPβCD using 1H-NMR and a solubility diagram was performed. A design of experiment was built to determine the optimal conditions to obtain a formulation that had the lowest turbidity and particle count. Our results showed that VA and CZ form an equimolar precipitate below pH 7.3. The best formulation obtained underwent an in-vitro evaluation of its antibacterial activity. The impact of HPCDs on incompatibility has been demonstrated through the inclusion of antibiotics and especially VA. The formulation has been shown to be able to inhibit the incompatibility for pH higher than 7.3 and to possess unaltered antibacterial activity.
Keywords: bacterial keratitis; ceftazidime; cyclodextrins; design of experiments; nuclear magnetic resonance; ophthalmic solution; vancomycin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

