Butyrylcholinesterase-Protein Interactions in Human Serum
- PMID: 34639003
- PMCID: PMC8508650
- DOI: 10.3390/ijms221910662
Butyrylcholinesterase-Protein Interactions in Human Serum
Abstract
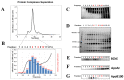
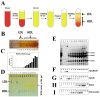
Measuring various biochemical and cellular components in the blood is a routine procedure in clinical practice. Human serum contains hundreds of diverse proteins secreted from all cells and tissues in healthy and diseased states. Moreover, some serum proteins have specific strong interactions with other blood components, but most interactions are probably weak and transient. One of the serum proteins is butyrylcholinesterase (BChE), an enzyme existing mainly as a glycosylated soluble tetramer that plays an important role in the metabolism of many drugs. Our results suggest that BChE interacts with plasma proteins and forms much larger complexes than predicted from the molecular weight of the BChE tetramer. To investigate and isolate such complexes, we developed a two-step strategy to find specific protein-protein interactions by combining native size-exclusion chromatography (SEC) with affinity chromatography with the resin that specifically binds BChE. Second, to confirm protein complexes' specificity, we fractionated blood serum proteins by density gradient ultracentrifugation followed by co-immunoprecipitation with anti-BChE monoclonal antibodies. The proteins coisolated in complexes with BChE were identified by mass spectroscopy. These binding studies revealed that BChE interacts with a number of proteins in the human serum. Some of these interactions seem to be more stable than transient. BChE copurification with ApoA-I and the density of some fractions containing BChE corresponding to high-density lipoprotein cholesterol (HDL) during ultracentrifugation suggest its interactions with HDL. Moreover, we observed lower BChE plasma activity in individuals with severely reduced HDL levels (≤20 mg/dL). The presented two-step methodology for determination of the BChE interactions can facilitate further analysis of such complexes, especially from the brain tissue, where BChE could be involved in the pathogenesis and progression of AD.
Keywords: ApoA-I; BChE; butyrylcholinesterase; high-density lipoprotein (HDL); protein interactions; pseudocholinesterase.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Burtis C.A., Ashwood E.R., Bruns D.E. Tietz, Fundamentals of Clinical Chemistry. 6th ed. Volume 54 Saunders/Elsevier; St. Louis, MO, USA: 2008.
-
- Rai A.J., Gelfand C.A., Haywood B.C., Warunek D.J., Yi J., Schuchard M.D., Mehigh R.J., Cockrill S.L., Scott G.B.I., Tammen H., et al. HUPO Plasma Proteome Project specimen collection and handling: Towards the standardization of parameters for plasma proteome samples. Proteomics. 2005;5:3262–3277. doi: 10.1002/pmic.200401245. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

