Chronic and Cycling Hypoxia: Drivers of Cancer Chronic Inflammation through HIF-1 and NF-κB Activation: A Review of the Molecular Mechanisms
- PMID: 34639040
- PMCID: PMC8509318
- DOI: 10.3390/ijms221910701
Chronic and Cycling Hypoxia: Drivers of Cancer Chronic Inflammation through HIF-1 and NF-κB Activation: A Review of the Molecular Mechanisms
Abstract
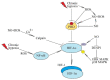
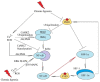
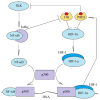
Chronic (continuous, non-interrupted) hypoxia and cycling (intermittent, transient) hypoxia are two types of hypoxia occurring in malignant tumors. They are both associated with the activation of hypoxia-inducible factor-1 (HIF-1) and nuclear factor κB (NF-κB), which induce changes in gene expression. This paper discusses in detail the mechanisms of activation of these two transcription factors in chronic and cycling hypoxia and the crosstalk between both signaling pathways. In particular, it focuses on the importance of reactive oxygen species (ROS), reactive nitrogen species (RNS) together with nitric oxide synthase, acetylation of HIF-1, and the action of MAPK cascades. The paper also discusses the importance of hypoxia in the formation of chronic low-grade inflammation in cancerous tumors. Finally, we discuss the effects of cycling hypoxia on the tumor microenvironment, in particular on the expression of VEGF-A, CCL2/MCP-1, CXCL1/GRO-α, CXCL8/IL-8, and COX-2 together with PGE2. These factors induce angiogenesis and recruit various cells into the tumor niche, including neutrophils and monocytes which, in the tumor, are transformed into tumor-associated neutrophils (TAN) and tumor-associated macrophages (TAM) that participate in tumorigenesis.
Keywords: HIF-1α; HIF-1β; NF-κB; cancer; cycling hypoxia; hypoxia-inducible factor; low-grade inflammation; tumor.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

