The NLRP3 Inflammasome: Relevance in Solid Organ Transplantation
- PMID: 34639062
- PMCID: PMC8509131
- DOI: 10.3390/ijms221910721
The NLRP3 Inflammasome: Relevance in Solid Organ Transplantation
Abstract
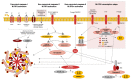
The NOD, LRR, and pyrin domain-containing 3 (NLRP3) protein has been established as a central component of the inflammasome and regulates the inflammatory response to a myriad of environmental, microbial, and endogenous danger stimuli. Assembly of the NLRP3 inflammasome results in the cleavage and activation of caspase-1, in turn causing release of the pro-inflammatory interleukins 1-beta and 18. This activation response, while crucial to coordinated innate immune defense, can be aberrantly activated by the likes of cell-free DNA, and cause significant autoimmune pathology. Complications of autoimmunity induced by aberrant NLRP3 inflammasome activation have a great degree of mechanistic crossover with alloimmune injury in solid organ transplant, and stratagems to neutralize NLRP3 inflammasome activation may prove beneficial in solid organ transplant management. This article reviews NLRP3 inflammasome biology and the pathology associated with its hyperactivation, as well as the connections between NLRP3 inflammasome activation and allograft homeostasis.
Keywords: NLRP3; alloimmune injury; autoimmunity; donor-derived cell-free DNA; fibrosis; inflammasome; neutrophil extracellular trap; solid organ transplant.
Conflict of interest statement
R.M.B., B.L.D. and S.D. are employees of CareDx, Inc.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

