Role of Human Primary Renal Fibroblast in TGF-β1-Mediated Fibrosis-Mimicking Devices
- PMID: 34639099
- PMCID: PMC8509581
- DOI: 10.3390/ijms221910758
Role of Human Primary Renal Fibroblast in TGF-β1-Mediated Fibrosis-Mimicking Devices
Abstract
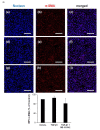
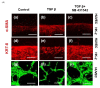
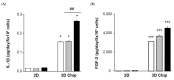
Renal fibrosis is a progressive chronic kidney disease that ultimately leads to end-stage renal failure. Despite several approaches to combat renal fibrosis, an experimental model to evaluate currently available drugs is not ideal. We developed fibrosis-mimicking models using three-dimensional (3D) co-culture devices designed with three separate layers of tubule interstitium, namely, epithelial, fibroblastic, and endothelial layers. We introduced human renal proximal tubular epithelial cells (HK-2), human umbilical-vein endothelial cells, and patient-derived renal fibroblasts, and evaluated the effects of transforming growth factor-β (TGF-β) and TGF-β inhibitor treatment on this renal fibrosis model. The expression of the fibrosis marker alpha smooth muscle actin upon TGF-β1 treatment was augmented in monolayer-cultured HK-2 cells in a 3D disease model. In the vascular compartment of renal fibrosis models, the density of vessels was increased and decreased in the TGF-β-treated group and TGF-β-inhibitor treatment group, respectively. Multiplex ELISA using supernatants in the TGF-β-stimulating 3D models showed that pro-inflammatory cytokine and growth factor levels including interleukin-1 beta, tumor necrosis factor alpha, basic fibroblast growth factor, and TGF-β1, TGF-β2, and TGF-β3 were increased, which mimicked the fibrotic microenvironments of human kidneys. This study may enable the construction of a human renal fibrosis-mimicking device model beyond traditional culture experiments.
Keywords: TGF-β1; fibrosis; renal fibroblast.
Conflict of interest statement
The authors declare no conflict of interest.
Figures










References
-
- Border W., Noble N. Transforming growth factor in tissue fibrosis. N. Engl. J. Med. 1994;331:1286–1292. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

