Anti-Hepatocellular Carcinoma Biomolecules: Molecular Targets Insights
- PMID: 34639131
- PMCID: PMC8509806
- DOI: 10.3390/ijms221910774
Anti-Hepatocellular Carcinoma Biomolecules: Molecular Targets Insights
Abstract
This report explores the available curative molecules directed against hepatocellular carcinoma (HCC). Limited efficiency as well as other drawbacks of existing molecules led to the search for promising potential alternatives. Understanding of the cell signaling mechanisms propelling carcinogenesis and driven by cell proliferation, invasion, and angiogenesis can offer valuable information for the investigation of efficient treatment strategies. The complexity of the mechanisms behind carcinogenesis inspires researchers to explore the ability of various biomolecules to target specific pathways. Natural components occurring mainly in food and medicinal plants, are considered an essential resource for discovering new and promising therapeutic molecules. Novel biomolecules normally have an advantage in terms of biosafety. They are also widely diverse and often possess potent antioxidant, anti-inflammatory, and anti-cancer properties. Based on quantitative structure-activity relationship studies, biomolecules can be used as templates for chemical modifications that improve efficiency, safety, and bioavailability. In this review, we focus on anti-HCC biomolecules that have their molecular targets partially or completely characterized as well as having anti-cancer molecular mechanisms that are fairly described.
Keywords: cancer; drug; hepatocellular carcinoma; molecular target; phytochemicals; signaling pathway.
Conflict of interest statement
The authors declare no conflict of interest.
Figures









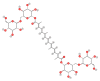
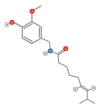
References
-
- Clark R., Lee S.-H. Anticancer properties of capsaicin against human cancer. Anticancer Res. 2016;36:837–843. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

