Saussurea involucrata (Snow Lotus) ICE1 and ICE2 Orthologues Involved in Regulating Cold Stress Tolerance in Transgenic Arabidopsis
- PMID: 34639192
- PMCID: PMC8509503
- DOI: 10.3390/ijms221910850
Saussurea involucrata (Snow Lotus) ICE1 and ICE2 Orthologues Involved in Regulating Cold Stress Tolerance in Transgenic Arabidopsis
Abstract
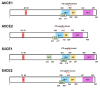
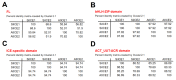
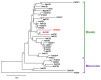
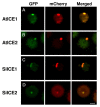
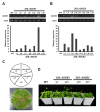
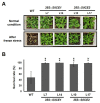
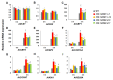
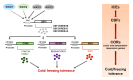
As with other environmental stresses, cold stress limits plant growth, geographical distribution, and agricultural productivity. CBF/DREB (CRT-binding factors/DRE-binding proteins) regulate tolerance to cold/freezing stress across plant species. ICE (inducer of CBF expression) is regarded as the upstream inducer of CBF expression and plays a crucial role as a main regulator of cold acclimation. Snow lotus (Saussurea involucrata) is a well-known traditional Chinese herb. This herb is known to have greater tolerance to cold/freezing stress compared to other plants. According to transcriptome datasets, two putative ICE homologous genes, SiICE1 and SiICE2, were identified in snow lotus. The predicted SiICE1 cDNA contains an ORF of 1506 bp, encoding a protein of 501 amino acids, whereas SiICE2 cDNA has an ORF of 1482 bp, coding for a protein of 493 amino acids. Sequence alignment and structure analysis show SiICE1 and SiICE2 possess a S-rich motif at the N-terminal region, while the conserved ZIP-bHLH domain and ACT domain are at the C-terminus. Both SiICE1 and SiICE2 transcripts were cold-inducible. Subcellular localization and yeast one-hybrid assays revealed that SiICE1 and SiICE2 are transcriptional regulators. Overexpression of SiICE1 (35S::SiICE1) and SiICE2 (35S::SiICE2) in transgenic Arabidopsis increased the cold tolerance. In addition, the expression patterns of downstream stress-related genes, CBF1, CBF2, CBF3, COR15A, COR47, and KIN1, were up-regulated when compared to the wild type. These results thus provide evidence that SiICE1 and SiICE2 function in cold acclimation and this cold/freezing tolerance may be regulated through a CBF-controlling pathway.
Keywords: CBF expression; ICE orthologues; cold stress tolerance; snow lotus.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Went F.W. The effect of temperature on plant growth. Annu. Rev. Plant Physiol. 1953;4:347–362. doi: 10.1146/annurev.pp.04.060153.002023. - DOI
-
- Hatfield J.L., Boote K.J., Kimball B.A., Ziska L.H., Izaurralde R.C., Ort D., Thomson A.M., Wolfe D.W. Climate impacts on agriculture: Implications for crop production. Agron. J. 2011;103:351–370. doi: 10.2134/agronj2010.0303. - DOI
-
- Barlow K.M., Christy B.P., O’Leary G.J., Riffkin P.A., Nuttall J.G. Simulating the impact of extreme heat and frost events on wheat crop production: A review. Field Crops Res. 2015;171:109–119. doi: 10.1016/j.fcr.2014.11.010. - DOI
-
- Hatfield J.L., Prueger J.H. Temperature extremes: Effect on plant growth and development. Weather Clim. Extrem. 2015;10:4–10. doi: 10.1016/j.wace.2015.08.001. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

