The Effect of Selective Retina Therapy with Automatic Real-Time Feedback-Controlled Dosimetry for Chronic Central Serous Chorioretinopathy: A Randomized, Open-Label, Controlled Clinical Trial
- PMID: 34640312
- PMCID: PMC8509340
- DOI: 10.3390/jcm10194295
The Effect of Selective Retina Therapy with Automatic Real-Time Feedback-Controlled Dosimetry for Chronic Central Serous Chorioretinopathy: A Randomized, Open-Label, Controlled Clinical Trial
Abstract
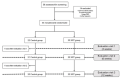
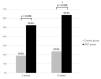
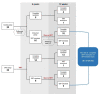
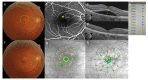
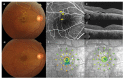
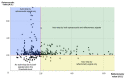
This prospective randomized controlled trial evaluated the safety and efficacy of real-time feedback-controlled dosimetry (RFD)-guided selective retina therapy (SRT) in chronic central serous chorioretinopathy (CSC). Forty-four participants with chronic CSC were included and randomly assigned to the control group or SRT group. The SRT laser system with RFD-guidance was applied to cover the entire leakage area. If SRF remained at the 6-week follow-up visit, re-treatment and rescue SRT was performed for the SRT group and crossover group, respectively. The rate of complete resolution of subretinal fluid (SRF), mean SRF height, and mean retinal sensitivity were compared between the two groups at 6-weeks post-treatment. The complete SRF resolution rate in all SRT-treated eyes was evaluated at 12-weeks post-treatment. The rate of complete SRF resolution was significantly higher in the SRT group (63.6%) than in the control group (23.8%) at 6-weeks post-treatment (p = 0.020). The mean SRF height at 6 weeks after SRT was significantly lower in the SRT group (p = 0.041). Overall, SRT-treated eyes showed complete SRF resolution in 70.3% of eyes at 12-weeks post-treatment. RFD-guided SRT was safe and effective to remove SRF in chronic CSC patients during the 3-month follow-up period.
Keywords: central serous chorioretinopathy; real-time feedback-controlled dosimetry; retinal sensitivity; selective retina therapy.
Conflict of interest statement
All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest and have disclosed the following: Young-Jung Roh have a patent related to real-time feedback dosimetry in South Korea. (Patent no.: 10-1966906). The SRT laser system (R:Gen, Lutronic) was approved for patients with central serous chorioretinopathy in South Korea. Lutronic provided the SRT laser system and technical support but had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures

References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

