Vaccination for Respiratory Infections in Patients with Heart Failure
- PMID: 34640328
- PMCID: PMC8509310
- DOI: 10.3390/jcm10194311
Vaccination for Respiratory Infections in Patients with Heart Failure
Abstract
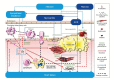
Bronchopulmonary infections are a major trigger of cardiac decompensation and are frequently associated with hospitalizations in patients with heart failure (HF). Adverse cardiac effects associated with respiratory infections, more specifically Streptococcus pneumoniae and influenza infections, are the consequence of inflammatory processes and thrombotic events. For both influenza and pneumococcal vaccinations, large multicenter randomized clinical trials are needed to evaluate their efficacy in preventing cardiovascular events, especially in HF patients. No study to date has evaluated the protective effect of the COVID-19 vaccine in patients with HF. Different guidelines recommend annual influenza vaccination for patients with established cardiovascular disease and also recommend pneumococcal vaccination in patients with HF. The Heart Failure group of the French Society of Cardiology recently strongly recommended vaccination against COVID-19 in HF patients. Nevertheless, the implementation of vaccination recommendations against respiratory infections in HF patients remains suboptimal. This suggests that a national health policy is needed to improve vaccination coverage, involving not only the general practitioner, but also other health providers, such as cardiologists, nurses, and pharmacists. This review first summarizes the pathophysiology of the interrelationships between inflammation, infection, and HF. Then, we describe the current clinical knowledge concerning the protective effect of vaccines against respiratory diseases (influenza, pneumococcal infection, and COVID-19) in patients with HF and finally we propose how vaccination coverage could be improved in these patients.
Keywords: COVID-19 vaccine; heart failure; influenza vaccine; pneumococcal vaccine; respiratory infections; review article; vaccination coverage.
Conflict of interest statement
N. Girerd reports personal fees from Astra-Zeneca, Bayer, Boehringer-Ingelheim, Novartis, Vifor and Lilly, outside the submitted work; N. Chapet reports personal fees from AstraZeneca, outside the submitted work; C. Roubille reports personal fees from Pfizer during the conduct of the study; personal fees from Servier and Pfizer, outside the submitted work; J. Roncalli reports personal fees from Pfizer during the conduct of the study; F. Mouquet reports personal fees from Bristol-Myers Squibb, AstraZeneca, non-financial support from Pfizer, personal fees and non-financial support from Novartis and Vifor, outside the submitted work; N. Lamblin reports personal fees from Actelion-Janssen, Amicus Therapeutics, AstraZeneca, Bayer, BMS, Boehringer-Ingelheim, MSD, Novartis, Pfizer and Sanofi-Aventis, outside the submitted work; J.P. Gueffet reports personal fees from CMD eHealth, Novartis, Novo Nordisk, non-financial support from AstraZeneca, outside the submitted work; T. Damy reports personal fees from Pfizer and Sanofi-Aventis during the conduct of the study. J.M. Tartiere reports personal fees, non-financial support and other from Novartis, personal fees and non-financial support from AstraZeneca, non-financial support and other from Amgen, other from Servier, Bayer, and Merck Sharp Dohme, during the conduct of the study; S. Aguilhon reports personal fees from AstraZeneca, Sanofi Genzyme, Pfizer outside the submitted work; R. Escamilla reports personal fees from Pfizer during the conduct of the study; F. Roubille reports grants, personal fees and non-financial support from Air Liquide, grants and personal fees from Abbott, Astrazeneca and Novartis, personal fees from Vifor, Servier, Abiomed, Zoll, Medtronic, Resmed, LVL, Eole, Pfizer, outside the submitted work; C. Janssen, M. Galinier, E. Berthelot and M. Salvat have nothing to disclose.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

