Current State of the Art and Prospects of T Cell-Redirecting Bispecific Antibodies in Multiple Myeloma
- PMID: 34640611
- PMCID: PMC8509238
- DOI: 10.3390/jcm10194593
Current State of the Art and Prospects of T Cell-Redirecting Bispecific Antibodies in Multiple Myeloma
Abstract
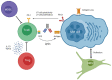
Multiple myeloma (MM) patients eventually develop multi-drug-resistant disease with poor survival. Hence, the development of novel treatment strategies is of great importance. Recently, different classes of immunotherapeutic agents have shown great promise in heavily pre-treated MM, including T cell-redirecting bispecific antibodies (BsAbs). These BsAbs simultaneously interact with CD3 on effector T cells and a tumor-associated antigen on MM cells, resulting in redirection of T cells to MM cells. This leads to the formation of an immunologic synapse, the release of granzymes/perforins, and subsequent tumor cell lysis. Several ongoing phase 1 studies show substantial activity and a favorable toxicity profile with BCMA-, GPRC5D-, or FcRH5-targeting BsAbs in heavily pre-treated MM patients. Resistance mechanisms against BsAbs include tumor-related features, T cell characteristics, and impact of components of the immunosuppressive tumor microenvironment. Various clinical trials are currently evaluating combination therapy with a BsAb and another agent, such as a CD38-targeting antibody or an immunomodulatory drug (e.g., pomalidomide), to further improve response depth and duration. Additionally, the combination of two BsAbs, simultaneously targeting two different antigens to prevent antigen escape, is being explored in clinical studies. The evaluation of BsAbs in earlier lines of therapy, including newly diagnosed MM, is warranted, based on the efficacy of BsAbs in advanced MM.
Keywords: BCMA; CD38; FcRH5; GPRC5D; bispecific antibody; multiple myeloma.
Conflict of interest statement
N.W.C.J.v.d.D. has received research support from Janssen Pharmaceuticals, AMGEN, Celgene, Novartis, Cellectis and BMS, and serves in advisory boards for Janssen Pharmaceuticals, AMGEN, Celgene, BMS, Takeda, Roche, Novartis, Bayer, Adaptive, GSK, and Servier; SZ serves in advisory boards for Celgene, Janssen Pharmaceuticals, Sanofi, Takeda, Amgen and has received research support from Janssen Pharmaceuticals, Takeda and Celgene. All other authors declared no conflicts of interest.
Figures

References
-
- Myeloma-Cancer Stat Facts. [(accessed on 25 June 2021)]; Available online: Seer.cancer.gov/statfacts/html/mulmy.html.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

