Fabrication of Novel and Potential Selective 4-Cyanophenol Chemical Sensor Probe Based on Cu-Doped Gd2O3 Nanofiber Materials Modified PEDOT:PSS Polymer Mixtures with Au/µ-Chip for Effective Monitoring of Environmental Contaminants from Various Water Samples
- PMID: 34641194
- PMCID: PMC8512155
- DOI: 10.3390/polym13193379
Fabrication of Novel and Potential Selective 4-Cyanophenol Chemical Sensor Probe Based on Cu-Doped Gd2O3 Nanofiber Materials Modified PEDOT:PSS Polymer Mixtures with Au/µ-Chip for Effective Monitoring of Environmental Contaminants from Various Water Samples
Abstract
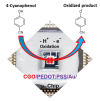
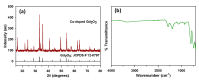
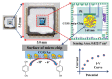
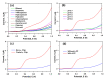
Herein, a novel copper-doped gadolinium oxide (Cu-doped Gd2O3; CGO) nanofiber was synthesized by a simple solution method in the basic phase and successfully characterized. We have used Fourier Transform Infrared Spectroscopy (FTIR), X-ray Diffraction (XRD), Field Emission Scanning Electron Microscope (FESEM) and Energy-Dispersive Spectroscopy (EDS) techniques for characterization of the CGO nanofiber. The CGO nanofiber was used later to modify Au-coated μ-Chips with poly(3,4-ethylenedioxythiophene) polystyrene sulfonate (PEDOT:PSS) polymer mixtures (coating binder) to selectively detect 4-cyanophenol (4-CP) in an aqueous medium. Notable sensing performance was achieved with excellent sensitivity (2.4214 µAµM-1 cm-2), fast response time (~12 s), wide linear dynamic range (LDR = 1.0 nM-1.0 mM: R2 = 0.9992), ultra-low detection limit (LoD; 1.3 ± 0.1 pM at S/N = 3), limit of quantification (LoQ; 4.33 pM), and excellent reproducibility and repeatability for CGO/Au/μ-Chip sensor. This CGO modified Au/μ-chip was further applied with appropriate quantification and determination results in real environmental sample analyses.
Keywords: 4-Cyanophenol; Cu-doped Gd2O3 nanofibers; PEDOT:PSS polymer matrixes; electrochemical method; real sample analysis; tiny Au/µ-Chip.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Development of Methanol Sensor Based on Sol-Gel Drop-Coating Co3O4·CdO·ZnO Nanoparticles Modified Gold-Coated µ-Chip by Electro-Oxidation Process.Gels. 2021 Nov 26;7(4):235. doi: 10.3390/gels7040235. Gels. 2021. PMID: 34940295 Free PMC article.
-
Sensitive Electrochemical Detection of 4-Nitrophenol with PEDOT:PSS Modified Pt NPs-Embedded PPy-CB@ZnO Nanocomposites.Biosensors (Basel). 2022 Nov 8;12(11):990. doi: 10.3390/bios12110990. Biosensors (Basel). 2022. PMID: 36354499 Free PMC article.
-
An alternative electrochemical approach for toluene detection with ZnO/MgO/Cr2O3 nanofibers on a glassy carbon electrode for environmental monitoring.RSC Adv. 2020 Dec 17;10(73):44641-44653. doi: 10.1039/d0ra08577d. eCollection 2020 Dec 17. RSC Adv. 2020. PMID: 35516258 Free PMC article.
-
Fabrication of an ultra-sensitive para-nitrophenol sensor based on facile Zn-doped Er2O3 nanocomposites via an electrochemical approach.Anal Methods. 2020 Jul 16;12(27):3470-3483. doi: 10.1039/d0ay00735h. Anal Methods. 2020. PMID: 32672282
-
Preparation and Characterization of PEDOT:PSS/TiO2 Micro/Nanofiber-Based Gas Sensors.Polymers (Basel). 2022 Apr 27;14(9):1780. doi: 10.3390/polym14091780. Polymers (Basel). 2022. PMID: 35566945 Free PMC article.
Cited by
-
4-Cyanophenol herbicide sensor using multi-walled carbon nanotube embedded dual-microporous polypyrrole nanoparticles as metal-free and environmentally friendly hybrid electrode.Mikrochim Acta. 2023 Apr 29;190(5):197. doi: 10.1007/s00604-023-05773-4. Mikrochim Acta. 2023. PMID: 37120457
References
-
- Forryan C.L., Lawrence N.S., Rees N.V., Compton R.G. Voltammetric Characterisation of the Radical Anions of 4-Nitrophenol, 2-Cyanophenol and 4-Cyanophenol in N,N-Dimethylformamide Electrogenerated at Gold Electrodes. J. Electroanal. Chem. 2004;561((Suppl. 1)):53–65. doi: 10.1016/j.jelechem.2003.07.001. - DOI
-
- Michałowicz J., Duda W. Phenols-Sources and Toxicity. Pol. J. Environ. Stud. 2007;16:347–362.
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

