Origin of Salt Effects in SN2 Fluorination Using KF Promoted by Ionic Liquids: Quantum Chemical Analysis
- PMID: 34641282
- PMCID: PMC8510065
- DOI: 10.3390/molecules26195738
Origin of Salt Effects in SN2 Fluorination Using KF Promoted by Ionic Liquids: Quantum Chemical Analysis
Abstract
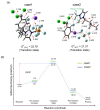
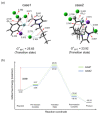
Quantum chemical analysis is presented, motivated by Grée and co-workers' observation of salt effects [Adv. Synth. Catal. 2006, 348, 1149-1153] for SN2 fluorination of KF in ionic liquids (ILs). We examine the relative promoting capacity of KF in [bmim]PF6 vs. [bmim]Cl by comparing the activation barriers of the reaction in the two ILs. We also elucidate the origin of the experimentally observed additional rate acceleration in IL [bmim]PF6 achieved by adding KPF6. We find that the anion PF6- in the added salt acts as an extra Lewis base binding to the counter-cation K+ to alleviate the strong Coulomb attractive force on the nucleophile F-, decreasing the Gibbs free energy of activation as compared with that in its absence, which is in good agreement with experimental observations of rate enhancement. We also predict that using 2 eq. KF together with an eq. KPF6 would further activate SN2 fluorination.
Keywords: SN2 fluorination; ionic liquid; mechanism; salt effect.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Potential of Mean Force Calculations for an SN2 Fluorination Reaction in Five Different Imidazolium Ionic Liquid Solvents Using Quantum Mechanics/Molecular Mechanics Molecular Dynamics Simulations.J Phys Chem B. 2020 May 28;124(21):4338-4357. doi: 10.1021/acs.jpcb.0c03192. Epub 2020 May 13. J Phys Chem B. 2020. PMID: 32352290
-
Inter- and Intra-Molecular Organocatalysis of SN2 Fluorination by Crown Ether: Kinetics and Quantum Chemical Analysis.Molecules. 2021 May 15;26(10):2947. doi: 10.3390/molecules26102947. Molecules. 2021. PMID: 34063489 Free PMC article.
-
Harnessing Ionic Interactions and Hydrogen Bonding for Nucleophilic Fluorination.Molecules. 2020 Feb 7;25(3):721. doi: 10.3390/molecules25030721. Molecules. 2020. PMID: 32046021 Free PMC article. Review.
-
SN2 fluorination reactions in ionic liquids: a mechanistic study towards solvent engineering.Org Biomol Chem. 2011 Jan 21;9(2):418-22. doi: 10.1039/c0ob00426j. Epub 2010 Oct 15. Org Biomol Chem. 2011. PMID: 20949216
-
Ionic liquids for nano- and microstructures preparation. Part 2: Application in synthesis.Adv Colloid Interface Sci. 2016 Jan;227:1-52. doi: 10.1016/j.cis.2015.08.010. Epub 2015 Aug 29. Adv Colloid Interface Sci. 2016. PMID: 26520242 Review.
Cited by
-
Ionic Liquids as Organocatalysts for Nucleophilic Fluorination: Concepts and Perspectives.Molecules. 2022 Sep 4;27(17):5702. doi: 10.3390/molecules27175702. Molecules. 2022. PMID: 36080470 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources

