Bruguiera gymnorhiza (L.) Lam. at the Forefront of Pharma to Confront Zika Virus and Microbial Infections-An In Vitro and In Silico Perspective
- PMID: 34641314
- PMCID: PMC8510246
- DOI: 10.3390/molecules26195768
Bruguiera gymnorhiza (L.) Lam. at the Forefront of Pharma to Confront Zika Virus and Microbial Infections-An In Vitro and In Silico Perspective
Abstract
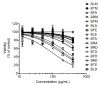
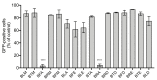
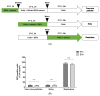
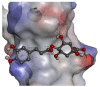
The recent emergence of Zika virus (ZIKV) in Brazil and the increasing resistance developed by pathogenic bacteria to nearly all existing antibiotics should be taken as a wakeup call for the international authority as this represents a risk for global public health. The lack of antiviral drugs and effective antibiotics on the market triggers the need to search for safe therapeutics from medicinal plants to fight viral and microbial infections. In the present study, we investigated whether a mangrove plant, Bruguiera gymnorhiza (L.) Lam. (B. gymnorhiza) collected in Mauritius, possesses antimicrobial and antibiotic potentiating abilities and exerts anti-ZIKV activity at non-cytotoxic doses. Microorganisms Escherichia coli ATCC 25922, Pseudomonas aeruginosa ATCC 27853, Klebsiella pneumoniae ATCC 70603, methicillin-resistant Staphylococcus aureus ATCC 43300 (MRSA), Salmonella enteritidis ATCC 13076, Sarcina lutea ATCC 9341, Proteus mirabilis ATCC 25933, Bacillus cereus ATCC 11778 and Candida albicans ATCC 26555 were used to evaluate the antimicrobial properties. Ciprofloxacin, chloramphenicol and streptomycin antibiotics were used for assessing antibiotic potentiating activity. ZIKVMC-MR766NIID (ZIKVGFP) was used for assessing anti-ZIKV activity. In silico docking (Autodock 4) and ADME (SwissADME) analyses were performed on collected data. Antimicrobial results revealed that Bruguiera twig ethyl acetate (BTE) was the most potent extract inhibiting the growth of all nine microbes tested, with minimum inhibitory concentrations ranging from 0.19-0.39 mg/mL. BTE showed partial synergy effects against MRSA and Pseudomonas aeruginosa when applied in combination with streptomycin and ciprofloxacin, respectively. By using a recombinant ZIKV-expressing reporter GFP protein, we identified both Bruguiera root aqueous and Bruguiera fruit aqueous extracts as potent inhibitors of ZIKV infection in human epithelial A549 cells. The mechanisms by which such extracts prevented ZIKV infection are linked to the inability of the virus to bind to the host cell surface. In silico docking showed that ZIKV E protein, which is involved in cell receptor binding, could be a target for cryptochlorogenic acid, a chemical compound identified in B. gymnorhiza. From ADME results, cryptochlorogenic acid is predicted to be not orally bioavailable because it is too polar. Scientific data collected in this present work can open a new avenue for the development of potential inhibitors from B. gymnorhiza to fight ZIKV and microbial infections in the future.
Keywords: ADME; antimicrobial; antiviral; envelope protein; mangrove plants; pharmacokinetics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Rhizophora mucronata Lam., a halophyte from Mauritius Island, inhibits the entry of Zika virus in human cells (A549)- an in vitro and in silico analysis.J Biomol Struct Dyn. 2023;41(22):12599-12609. doi: 10.1080/07391102.2023.2167115. Epub 2023 Jan 17. J Biomol Struct Dyn. 2023. PMID: 36648248
-
Antimicrobial and anti-Quorum Sensing activities of selected medicinal plants of Ethiopia: Implication for development of potent antimicrobial agents.BMC Microbiol. 2016 Jul 11;16(1):139. doi: 10.1186/s12866-016-0765-9. BMC Microbiol. 2016. PMID: 27400878 Free PMC article.
-
Phenolic compounds and biological activity of Kitaibelia vitifolia.J Med Food. 2011 Dec;14(12):1617-23. doi: 10.1089/jmf.2011.0013. Epub 2011 Aug 30. J Med Food. 2011. PMID: 21877950
-
Antimicrobial Tolerance in Cross-Kingdom Dual-Species Biofilms Formed by Fungi and Bacteria.Med Mycol J. 2024;65(3):49-57. doi: 10.3314/mmj.24.004. Med Mycol J. 2024. PMID: 39218647 Review.
-
Design, synthesis, and molecular modeling studies of novel 2-quinolone-1,2,3-triazole-α-aminophosphonates hybrids as dual antiviral and antibacterial agents.Eur J Med Chem. 2024 Mar 15;268:116235. doi: 10.1016/j.ejmech.2024.116235. Epub 2024 Feb 16. Eur J Med Chem. 2024. PMID: 38377828 Review.
Cited by
-
Natural Products and Derivatives as Potential Zika virus Inhibitors: A Comprehensive Review.Viruses. 2023 May 20;15(5):1211. doi: 10.3390/v15051211. Viruses. 2023. PMID: 37243296 Free PMC article. Review.
-
Exploring the Mangrove Fruit: From the Phytochemicals to Functional Food Development and the Current Progress in the Middle East.Mar Drugs. 2022 Apr 28;20(5):303. doi: 10.3390/md20050303. Mar Drugs. 2022. PMID: 35621954 Free PMC article. Review.
-
Antiviral Effect of Stenocline ericoides DC. and Stenocline inuloides DC., Two Flavonoid-Rich Endemic Plants from Madagascar, against Dengue and Zika Viruses.Pharmaceuticals (Basel). 2022 Nov 30;15(12):1500. doi: 10.3390/ph15121500. Pharmaceuticals (Basel). 2022. PMID: 36558951 Free PMC article.
-
Antimicrobial Secondary Metabolites from the Mangrove Plants of Asia and the Pacific.Mar Drugs. 2022 Oct 15;20(10):643. doi: 10.3390/md20100643. Mar Drugs. 2022. PMID: 36286466 Free PMC article. Review.
-
Chemical Constituents and Biological Activities of Bruguiera Genus and Its Endophytes: A Review.Mar Drugs. 2024 Mar 29;22(4):158. doi: 10.3390/md22040158. Mar Drugs. 2024. PMID: 38667775 Free PMC article. Review.
References
-
- Dos Santos Ramos M.A., dos Santos K.C., da Silva P.B., de Toledo L.G., Marena G.D., Rodero C.F., de Camargo B.A.F., Fortunato G.C., Bauab T.M., Chorilli M. Nanotechnological strategies for systemic microbial infections treatment: A review. Int. J. Pharm. 2020;589:119780. doi: 10.1016/j.ijpharm.2020.119780. - DOI - PMC - PubMed
-
- Sadeer N.B., Mahomoodally F. Antibiotic potentiation of natural products: A promising target to fight pathogenic bacteria. Curr. Drug Targets. 2020;21:1–17. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

