Validation of Structural Grounds for Anomalous Molecular Mobility in Ionic Liquid Glasses
- PMID: 34641371
- PMCID: PMC8510339
- DOI: 10.3390/molecules26195828
Validation of Structural Grounds for Anomalous Molecular Mobility in Ionic Liquid Glasses
Abstract
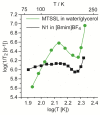
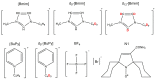
Ionic liquid (IL) glasses have recently drawn much interest as unusual media with unique physicochemical properties. In particular, anomalous suppression of molecular mobility in imidazolium IL glasses vs. increasing temperature was evidenced by pulse Electron Paramagnetic Resonance (EPR) spectroscopy. Although such behavior has been proven to originate from dynamics of alkyl chains of IL cations, the role of electron spin relaxation induced by surrounding protons still remains unclear. In this work we synthesized two deuterated imidazolium-based ILs to reduce electron-nuclear couplings between radical probe and alkyl chains of IL, and investigated molecular mobility in these glasses. The obtained trends were found closely similar for deuterated and protonated analogs, thus excluding the relaxation-induced artifacts and reliably demonstrating structural grounds of the observed anomalies in heterogeneous IL glasses.
Keywords: deuteration effect; glasses; ionic liquids; molecular mobility; nanostructure; spin probe.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Xu C., Cheng Z. Thermal stability of ionic liquids: Current status and prospects for future development. Processes. 2021;9:337. doi: 10.3390/pr9020337. - DOI
-
- Wu H.B., Zhang B., Liu S.H., Chen C.C. Flammability estimation of 1-hexyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide. J. Loss Prev. Process. Ind. 2020;66:104196. doi: 10.1016/j.jlp.2020.104196. - DOI
-
- Barulli L., Mezzetta A., Brunetti B., Guazzelli L., Vecchio Ciprioti S., Ciccioli A. Evaporation thermodynamics of the tetraoctylphosphonium bis(trifluoromethansulfonyl)imide([P8888]NTf2) and tetraoctylphosphonium nonafluorobutane-1-sulfonate ([P8888]NFBS) ionic liquids. J. Mol. Liq. 2021;333 doi: 10.1016/j.molliq.2021.115892. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

