Identification of the Protein Glycation Sites in Human Myoglobin as Rapidly Induced by d-Ribose
- PMID: 34641382
- PMCID: PMC8512392
- DOI: 10.3390/molecules26195829
Identification of the Protein Glycation Sites in Human Myoglobin as Rapidly Induced by d-Ribose
Abstract
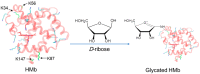
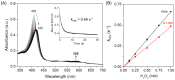
Protein glycation is an important protein post-translational modification and is one of the main pathogenesis of diabetic angiopathy. Other than glycated hemoglobin, the protein glycation of other globins such as myoglobin (Mb) is less studied. The protein glycation of human Mb with ribose has not been reported, and the glycation sites in the Mb remain unknown. This article reports that d-ribose undergoes rapid protein glycation of human myoglobin (HMb) at lysine residues (K34, K87, K56, and K147) on the protein surface, as identified by ultra-high performance liquid chromatography-mass spectrometry (UHPLC-MS) and electrospray ionization tandem mass spectrometry (ESI-MS/MS). Moreover, glycation by d-ribose at these sites slightly decreased the rate of the met heme (FeIII) in reaction with H2O2 to form a ferryl heme (FeIV=O). This study provides valuable insight into the protein glycation by d-ribose and provides a foundation for studying the structure and function of glycated heme proteins.
Keywords: d-ribose; diabetes; glycosylation sites; human myoglobin; protein glycation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Schalkwijk C.G., Ligtvoet N., Twaalfhoven H., Jager A., Blaauwgeers H.G., Schlingemann R.O., Tarnow L., Parving H.H., Stehouwer C.D., Van Hinsbergh V.W. Amadori albumin in type 1 diabetic patients: Correlation with markers of endothelial function, association with diabetic nephropathy, and localization in retinal capillaries. Diabetes. 1999;48:2446–2453. doi: 10.2337/diabetes.48.12.2446. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

