An Insight into FDA Approved Antibody-Drug Conjugates for Cancer Therapy
- PMID: 34641391
- PMCID: PMC8510272
- DOI: 10.3390/molecules26195847
An Insight into FDA Approved Antibody-Drug Conjugates for Cancer Therapy
Abstract
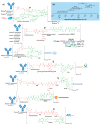
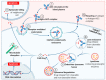
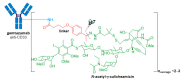
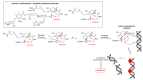
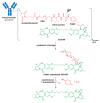
The large number of emerging antibody-drug conjugates (ADCs) for cancer therapy has resulted in a significant market 'boom', garnering worldwide attention. Despite ADCs presenting huge challenges to researchers, particularly regarding the identification of a suitable combination of antibody, linker, and payload, as of September 2021, 11 ADCs have been granted FDA approval, with eight of these approved since 2017 alone. Optimism for this therapeutic approach is clear, despite the COVID-19 pandemic, 2020 was a landmark year for deals and partnerships in the ADC arena, suggesting that there remains significant interest from Big Pharma. Herein we review the enthusiasm for ADCs by focusing on the features of those approved by the FDA, and offer some thoughts as to where the field is headed.
Keywords: ADCs; FDA approved; antibody-drug conjugates; cancer; targeted therapy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

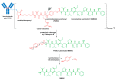


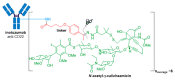
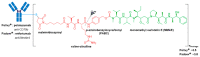
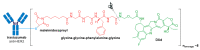


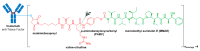
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

