Crystal Structure and Solid-State Packing of 4-Chloro-5 H-1,2,3-dithiazol-5-one and 4-Chloro-5 H-1,2,3-dithiazole-5-thione
- PMID: 34641419
- PMCID: PMC8512425
- DOI: 10.3390/molecules26195875
Crystal Structure and Solid-State Packing of 4-Chloro-5 H-1,2,3-dithiazol-5-one and 4-Chloro-5 H-1,2,3-dithiazole-5-thione
Abstract
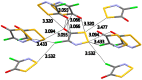
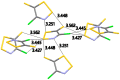
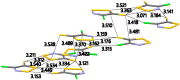
The crystal structure and solid-state packing of 4-chloro-5H-1,2,3-dithiazol-5-one and two polymorphs of 4-chloro-5H-1,2,3-dithiazole-5-thione were analyzed and compared to structural data of similar systems. These five-membered S,N-rich heterocycles are planar with considerable bond localization. All three structures demonstrate tight solid-state packing without voids which is attributed to a rich network of short intermolecular electrostatic contacts. These include Sδ+…Nδ-, Sδ+…Oδ-, Sδ+…Clδ- and Sδ+…Sδ- interactions that are well within the sum of their van der Waals radii (∑VDW). B3LYP, BLYP, M06, mPW1PW, PBE and MP2 were employed to calculate their intramolecular geometrical parameters, the Fukui condensed functions to probe their reactivity, the bond order, Bird Index and NICS(1) to establish their aromaticity.
Keywords: 1,2,3-dithiazol-5-one; 1,2,3-dithiazole-5-thione; 1,2,3-dithiazoles; Appel’s salt; dithiazines; thiazoles.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Appel R., Janssen H., Siray M., Knoch F. Synthese und reaktionen des 4,5-dichlor-1,2,3-dithiazoliumchlorids. Chem. Ber. 1985;118:1632–1643. doi: 10.1002/cber.19851180430. - DOI
-
- Kim K. Recent Advances in 1,2,3-Dithiazole Chemistry. Sulfur Silicon Relat. Elem. 1997;120:229–244. doi: 10.1080/10426509708545521. - DOI
-
- Kim K. Synthesis and Reactions of 1,2,3-Dithiazoles. Sulfur Rep. 1998;21:147–207. doi: 10.1080/01961779808047935. - DOI
-
- Konstantinova L.S., Rakitin O.A. Synthesis and properties of 1,2,3-dithiazoles. Russ. Chem. Rev. 2008;77:521–546. doi: 10.1070/RC2008v077n06ABEH003784. - DOI
LinkOut - more resources
Full Text Sources

