A Systematic Review of the Biological Effects of Cordycepin
- PMID: 34641429
- PMCID: PMC8510467
- DOI: 10.3390/molecules26195886
A Systematic Review of the Biological Effects of Cordycepin
Abstract
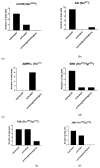
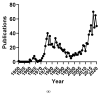
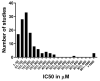
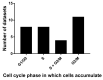
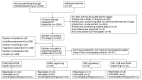
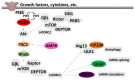
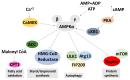
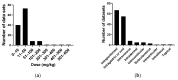
We conducted a systematic review of the literature on the effects of cordycepin on cell survival and proliferation, inflammation, signal transduction and animal models. A total of 1204 publications on cordycepin were found by the cut-off date of 1 February 2021. After application of the exclusion criteria, 791 papers remained. These were read and data on the chosen subjects were extracted. We found 192 papers on the effects of cordycepin on cell survival and proliferation and calculated a median inhibitory concentration (IC50) of 135 µM. Cordycepin consistently repressed cell migration (26 papers) and cellular inflammation (53 papers). Evaluation of 76 papers on signal transduction indicated consistently reduced PI3K/mTOR/AKT and ERK signalling and activation of AMPK. In contrast, the effects of cordycepin on the p38 and Jun kinases were variable, as were the effects on cell cycle arrest (53 papers), suggesting these are cell-specific responses. The examination of 150 animal studies indicated that purified cordycepin has many potential therapeutic effects, including the reduction of tumour growth (37 papers), repression of pain and inflammation (9 papers), protecting brain function (11 papers), improvement of respiratory and cardiac conditions (8 and 19 papers) and amelioration of metabolic disorders (8 papers). Nearly all these data are consistent with cordycepin mediating its therapeutic effects through activating AMPK, inhibiting PI3K/mTOR/AKT and repressing the inflammatory response. We conclude that cordycepin has excellent potential as a lead for drug development, especially for age-related diseases. In addition, we discuss the remaining issues around the mechanism of action, toxicity and biodistribution of cordycepin.
Keywords: AKT; AMPK; ERK; cell viability; cordycepin; inflammation; mTOR; natural product; review; signal transduction.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Winkler D. Cordyceps sinensis—a precious parasitic fungus infecting Tibet. Field Mycol. 2010;11:60. doi: 10.1016/j.fldmyc.2010.04.009. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

