Advances of Microneedles in Biomedical Applications
- PMID: 34641460
- PMCID: PMC8512585
- DOI: 10.3390/molecules26195912
Advances of Microneedles in Biomedical Applications
Abstract
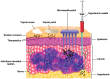
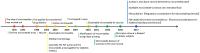
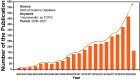
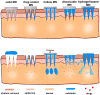
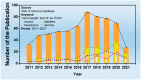
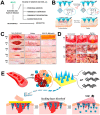
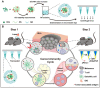
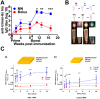
A microneedle (MN) is a painless and minimally invasive drug delivery device initially developed in 1976. As microneedle technology evolves, microneedles with different shapes (cone and pyramid) and forms (solid, drug-coated, hollow, dissolvable and hydrogel-based microneedles) have been developed. The main objective of this review is the applications of microneedles in biomedical areas. Firstly, the classifications and manufacturing of microneedle are briefly introduced so that we can learn the advantages and fabrications of different MNs. Secondly, research of microneedles in biomedical therapy such as drug delivery systems, diagnoses of disease, as well as wound repair and cancer therapy are overviewed. Finally, the safety and the vision of the future of MNs are discussed.
Keywords: biomedical application; classification; manufacture; microneedle; pitfalls.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

