Total Syntheses of Pladienolide-Derived Spliceosome Modulators
- PMID: 34641481
- PMCID: PMC8512135
- DOI: 10.3390/molecules26195938
Total Syntheses of Pladienolide-Derived Spliceosome Modulators
Abstract
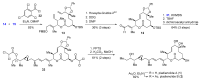
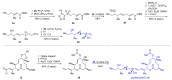
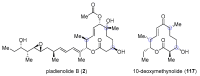
Pladienolides, an emerging class of naturally occurring spliceosome modulators, exhibit interesting structural features, such as highly substituted 12-membered macrocycles and epoxide-containing diene side chains. The potential of pladienolides as anti-cancer agents is confirmed by H3B-8800, a synthetic analog of this natural product class, which is currently under Phase I clinical trials. Since its isolation in 2004 and the first total synthesis in 2007, a dozen total syntheses and synthetic approaches toward the pladienolide class have been reported to date. This review focuses on the eight completed total syntheses of naturally occurring pladienolides or their synthetic analogs, in addition to a synthetic approach to the main framework of the natural product.
Keywords: macrocycles; natural products; pladienolide class; spliceosome; total synthesis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
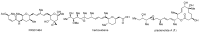


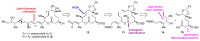

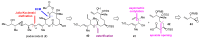
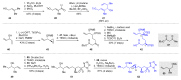
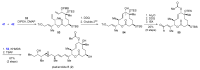
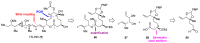





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

