Alpha-Glucosidase Inhibitory Assay-Screened Isolation and Molecular Docking Model from Bauhinia pulla Active Compounds
- PMID: 34641514
- PMCID: PMC8512368
- DOI: 10.3390/molecules26195970
Alpha-Glucosidase Inhibitory Assay-Screened Isolation and Molecular Docking Model from Bauhinia pulla Active Compounds
Abstract
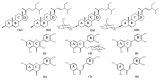
The aim of this research was to establish the constituents of Bauhinia pulla as anti-diabetic agents. A phytochemistry analysis was conducted by chromatographic and spectroscopic techniques. The alpha-glucosidase inhibitory assay screening resulted in the isolation of eight known compounds of quercetin, quercitrin, luteolin, 5-deoxyluteolin, 4-methyl ether isoliquiritigenin, 3,2',4'-trihydroxy-4-methoxychalcone, stigmasterol and β-sitosterol. Ethanol leaf extracts showed potential effects, which led to a strong inhibitory activity of isolated quercetin at 138.95 µg/mL and 5.41 µg/mL of IC50, respectively. The docking confirmed that flavonoids and chalcones had the same potential binding sites and responsibilities for their activity. This study was the first report of Bauhinia pulla chemical constituents and its alpha-glucosidase inhibition.
Keywords: Bauhinia pulla; alpha-glucosidase inhibition; anti-diabetic; molecular docking.
Conflict of interest statement
We declare there is no conflict of interest.
Figures

Similar articles
-
Bauhinia pulchella: chemical constituents, antioxidant and alpha-glucosidase inhibitory activities.Nat Prod Res. 2022 Mar;36(6):1604-1609. doi: 10.1080/14786419.2021.1887176. Epub 2021 Feb 13. Nat Prod Res. 2022. PMID: 33586542
-
Two new sesquiterpenol caffeates from Bauhinia brychycarpa Wall. ex Benth. and their α-glucosidase inhibitory activity.Fitoterapia. 2025 Jan;180:106327. doi: 10.1016/j.fitote.2024.106327. Epub 2024 Dec 4. Fitoterapia. 2025. PMID: 39643164
-
In silico assessment of potential leads identified from Bauhinia rufescens Lam. as α-glucosidase and α-amylase inhibitors.J Recept Signal Transduct Res. 2021 Apr;41(2):159-169. doi: 10.1080/10799893.2020.1800734. Epub 2020 Jul 27. J Recept Signal Transduct Res. 2021. PMID: 32718219
-
Acylated glucosylflavones as α-glucosidase inhibitors from Tinospora crispa leaf.Bioorg Med Chem. 2015 Jul 1;23(13):3388-96. doi: 10.1016/j.bmc.2015.04.053. Epub 2015 May 5. Bioorg Med Chem. 2015. PMID: 25999202
-
Unveiling Natural and Semisynthetic Acylated Flavonoids: Chemistry and Biological Actions in the Context of Molecular Docking.Molecules. 2022 Aug 26;27(17):5501. doi: 10.3390/molecules27175501. Molecules. 2022. PMID: 36080269 Free PMC article. Review.
Cited by
-
Synthesis of Novel N-Methylmorpholine-Substituted Benzimidazolium Salts as Potential α-Glucosidase Inhibitors.Molecules. 2022 Sep 15;27(18):6012. doi: 10.3390/molecules27186012. Molecules. 2022. PMID: 36144750 Free PMC article.
-
Flavonoid Constituents and Alpha-Glucosidase Inhibition of Solanum stramonifolium Jacq. Inflorescence with In Vitro and In Silico Studies.Molecules. 2022 Nov 24;27(23):8189. doi: 10.3390/molecules27238189. Molecules. 2022. PMID: 36500280 Free PMC article.
-
Alpha-Glucosidase Inhibition and Molecular Docking of Isolated Compounds from Traditional Thai Medicinal Plant, Neuropeltis racemosa Wall.Molecules. 2022 Jan 19;27(3):639. doi: 10.3390/molecules27030639. Molecules. 2022. PMID: 35163903 Free PMC article.
-
Exploring sesquiterpene lactone as a dual therapeutic agent for diabetes and oxidative stress: insights into PI3K/AKT modulation.3 Biotech. 2024 Sep;14(9):205. doi: 10.1007/s13205-024-04050-2. Epub 2024 Aug 19. 3 Biotech. 2024. PMID: 39170770 Free PMC article.
-
Alpha-glucosidase inhibitory activities of astilbin contained in Bauhinia strychnifolia Craib. stems: an investigation by in silico and in vitro studies.BMC Complement Med Ther. 2023 Jan 30;23(1):25. doi: 10.1186/s12906-023-03857-5. BMC Complement Med Ther. 2023. PMID: 36717857 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

