Dendrimers as Non-Viral Vectors in Gene-Directed Enzyme Prodrug Therapy
- PMID: 34641519
- PMCID: PMC8512881
- DOI: 10.3390/molecules26195976
Dendrimers as Non-Viral Vectors in Gene-Directed Enzyme Prodrug Therapy
Abstract
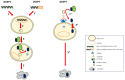
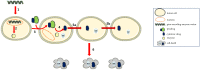
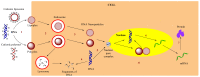
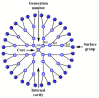
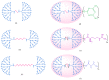
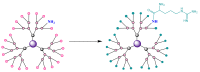
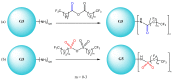
Gene-directed enzyme prodrug therapy (GDEPT) has been intensively studied as a promising new strategy of prodrug delivery, with its main advantages being represented by an enhanced efficacy and a reduced off-target toxicity of the active drug. In recent years, numerous therapeutic systems based on GDEPT strategy have entered clinical trials. In order to deliver the desired gene at a specific site of action, this therapeutic approach uses vectors divided in two major categories, viral vectors and non-viral vectors, with the latter being represented by chemical delivery agents. There is considerable interest in the development of non-viral vectors due to their decreased immunogenicity, higher specificity, ease of synthesis and greater flexibility for subsequent modulations. Dendrimers used as delivery vehicles offer many advantages, such as: nanoscale size, precise molecular weight, increased solubility, high load capacity, high bioavailability and low immunogenicity. The aim of the present work was to provide a comprehensive overview of the recent advances regarding the use of dendrimers as non-viral carriers in the GDEPT therapy.
Keywords: GDEP therapy; GDEPT; delivery vehicles; dendrimer; gene delivery system; non-viral vector; targeted therapy; transgene.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Sirhan J., Karaman R. Prodrugs Design A New Era. Nova Science Publishers, Inc.; New York, NY, USA: 2014. Gene directed enzyme prodrug therapy (GDEPT) pp. 210–232.
-
- Niculescu-Duvaz I., Springer C.J. Antibody-directed enzyme prodrug therapy (ADEPT): A targeting strategy in cancer chemotherapy. Curr. Med. Chem. 1995;2:687–706.
-
- Phillips G.L. Antibody-Drug Conjugates and Immunotoxins: From Pre-Clinical Development to Therapeutic Applications. Springer; New York, NY, USA: 2013.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

