Biginelli Reaction Mediated Synthesis of Antimicrobial Pyrimidine Derivatives and Their Therapeutic Properties
- PMID: 34641566
- PMCID: PMC8512088
- DOI: 10.3390/molecules26196022
Biginelli Reaction Mediated Synthesis of Antimicrobial Pyrimidine Derivatives and Their Therapeutic Properties
Abstract
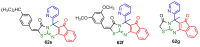
Antimicrobial resistance was one of the top priorities for global public health before the start of the 2019 coronavirus pandemic (COVID-19). Moreover, in this changing medical landscape due to COVID-19, finding new organic structures with antimicrobial and antiviral properties is a priority in current research. The Biginelli synthesis that mediates the production of pyrimidine compounds has been intensively studied in recent decades, especially due to the therapeutic properties of the resulting compounds, such as calcium channel blockers, anticancer, antiviral, antimicrobial, anti-inflammatory or antioxidant compounds. In this review we aim to review the Biginelli syntheses reported recently in the literature that mediates the synthesis of antimicrobial compounds, the spectrum of their medicinal properties, and the structure-activity relationship in the studied compounds.
Keywords: Biginelli reaction; antimicrobials; antioxidant; antitubercular; catalyst; dihydropyrimidininones.
Conflict of interest statement
The author declares no conflict of interest.
Figures












































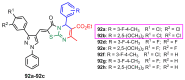


Similar articles
-
Synthesis and QSAR studies of pyrimido[4,5-d]pyrimidine-2,5-dione derivatives as potential antimicrobial agents.Bioorg Med Chem Lett. 2004 Aug 16;14(16):4185-90. doi: 10.1016/j.bmcl.2004.06.014. Bioorg Med Chem Lett. 2004. PMID: 15261267
-
Pyridine Compounds with Antimicrobial and Antiviral Activities.Int J Mol Sci. 2022 May 18;23(10):5659. doi: 10.3390/ijms23105659. Int J Mol Sci. 2022. PMID: 35628466 Free PMC article. Review.
-
Green synthesis and antimicrobial evaluation of some new trifluoromethyl-substituted hexahydropyrimidines by grinding.Eur J Med Chem. 2011 Nov;46(11):5636-40. doi: 10.1016/j.ejmech.2011.09.036. Epub 2011 Sep 29. Eur J Med Chem. 2011. PMID: 22000920
-
Efficient one-pot preparation of novel fused chromeno[2,3-d]pyrimidine and pyrano[2,3-d]pyrimidine derivatives.Eur J Med Chem. 2012 Jan;47(1):18-23. doi: 10.1016/j.ejmech.2011.09.040. Epub 2011 Oct 1. Eur J Med Chem. 2012. PMID: 22000923
-
Thieno[2,3-d]pyrimidine as a promising scaffold in medicinal chemistry: Recent advances.Bioorg Med Chem. 2019 Apr 1;27(7):1159-1194. doi: 10.1016/j.bmc.2019.02.044. Epub 2019 Feb 21. Bioorg Med Chem. 2019. PMID: 30826188 Review.
Cited by
-
O,S,Se-containing Biginelli products based on cyclic β-ketosulfone and their postfunctionalization.Beilstein J Org Chem. 2024 Aug 27;20:2143-2151. doi: 10.3762/bjoc.20.184. eCollection 2024. Beilstein J Org Chem. 2024. PMID: 39224228 Free PMC article.
-
Synthesis of Dihydropyrimidines: Isosteres of Nifedipine and Evaluation of Their Calcium Channel Blocking Efficiency.Molecules. 2023 Jan 12;28(2):784. doi: 10.3390/molecules28020784. Molecules. 2023. PMID: 36677842 Free PMC article. Review.
-
Synthesis of 3,4-Dihydropyrimidin(thio)one Containing Scaffold: Biginelli-like Reactions.Pharmaceuticals (Basel). 2022 Jul 30;15(8):948. doi: 10.3390/ph15080948. Pharmaceuticals (Basel). 2022. PMID: 36015096 Free PMC article. Review.
-
Design, Synthesis, Molecular Modeling, and Biological Evaluation of Novel Pyrimidine Derivatives as Potential Calcium Channel Blockers.Molecules. 2023 Jun 20;28(12):4869. doi: 10.3390/molecules28124869. Molecules. 2023. PMID: 37375424 Free PMC article.
-
Unravelling the potency of the 4-oxo-2-thioxo-1,2,3,4-tetrahydropyrimidine-5-carbonitrile scaffold with S-arylamide hybrids as PIM-1 kinase inhibitors: synthesis, biological activity and in silico studies.RSC Med Chem. 2025 Mar 28. doi: 10.1039/d5md00021a. Online ahead of print. RSC Med Chem. 2025. PMID: 40162200 Free PMC article.
References
-
- Hantzsch A. Condensations produkte aus Aldehydammoniak und ketonartigen Verbindungen. Ber. Der Dtsch. Chem. Ges. 1881;14:1637–1638. doi: 10.1002/cber.18810140214. - DOI
-
- Biginelli P. Aldehyde-urea derivatives of aceto-and oxaloacetic acids. Gazz. Chim. Ital. 1893;23:360–413.
-
- Kappe C.O. 100 years of the Biginelli dihydropyrimidine synthesis. Tetrahedron. 1993;49:6937–6963. doi: 10.1016/S0040-4020(01)87971-0. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

