Fluorescent Liquid Tetrazines
- PMID: 34641592
- PMCID: PMC8512366
- DOI: 10.3390/molecules26196047
Fluorescent Liquid Tetrazines
Abstract
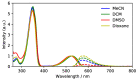
Tetrazines with branched alkoxy substituents are liquids at ambient temperature that despite the high chromophore density retain the bright orange fluorescence that is characteristic of this exceptional fluorophore. Here, we study the photophysical properties of a series of alkoxy-tetrazines in solution and as neat liquids. We also correlate the size of the alkoxy substituents with the viscosity of the liquids. We show using time-resolved spectroscopy that intersystem crossing is an important decay pathway competing with fluorescence, and that its rate is higher for 3,6-dialkoxy derivatives than for 3-chloro-6-alkoxytetrazines, explaining the higher fluorescence quantum yields for the latter. Quantum chemical calculations suggest that the difference in rate is due to the activation energy required to distort the tetrazine core such that the nπ*S1 and the higher-lying ππ*T2 states cross, at which point the spin-orbit coupling exceeding 10 cm-1 allows for efficient intersystem crossing to occur. Femtosecond time-resolved anisotropy studies in solution allow us to measure a positive relationship between the alkoxy chain lengths and their rotational correlation times, and studies in the neat liquids show a fast decay of the anisotropy consistent with fast exciton migration in the neat liquid films.
Keywords: excited states; fluorescence; inter system crossing; rheology; viscosity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Lu F., Nakanishi T. Solvent-Free Luminous Molecular Liquids. Adv. Opt. Mater. 2019;7:1900176. doi: 10.1002/adom.201900176. - DOI
-
- Kushwaha K., Yu L., Stranius K., Singh S., Hultmark S., Iqbal M., Eriksson L., Johnston E., Erhart P., Müller C., et al. A Record Chromophore Density in High-Entropy Liquids of Two Low-Melting Perylenes: A New Strategy for Liquid Chromophores. Adv. Sci. 2019;6:1801650. doi: 10.1002/advs.201801650. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

