In Vitro Analyses of Spinach-Derived Opioid Peptides, Rubiscolins: Receptor Selectivity and Intracellular Activities through G Protein- and β-Arrestin-Mediated Pathways
- PMID: 34641621
- PMCID: PMC8513079
- DOI: 10.3390/molecules26196079
In Vitro Analyses of Spinach-Derived Opioid Peptides, Rubiscolins: Receptor Selectivity and Intracellular Activities through G Protein- and β-Arrestin-Mediated Pathways
Abstract
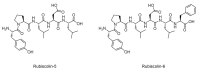
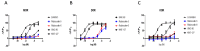
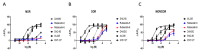
Activated opioid receptors transmit internal signals through two major pathways: the G-protein-mediated pathway, which exerts analgesia, and the β-arrestin-mediated pathway, which leads to unfavorable side effects. Hence, G-protein-biased opioid agonists are preferable as opioid analgesics. Rubiscolins, the spinach-derived naturally occurring opioid peptides, are selective δ opioid receptor agonists, and their p.o. administration exhibits antinociceptive effects. Although the potency and effect of rubiscolins as G-protein-biased molecules are partially confirmed, their in vitro profiles remain unclear. We, therefore, evaluated the properties of rubiscolins, in detail, through several analyses, including the CellKeyTM assay, cADDis® cAMP assay, and PathHunter® β-arrestin recruitment assay, using cells stably expressing µ, δ, κ, or µ/δ heteromer opioid receptors. In the CellKeyTM assay, rubiscolins showed selective agonistic effects for δ opioid receptor and little agonistic or antagonistic effects for µ and κ opioid receptors. Furthermore, rubiscolins were found to be G-protein-biased δ opioid receptor agonists based on the results obtained in cADDis® cAMP and PathHunter® β-arrestin recruitment assays. Finally, we found, for the first time, that they are also partially agonistic for the µ/δ dimers. In conclusion, rubiscolins could serve as attractive seeds, as δ opioid receptor-specific agonists, for the development of novel opioid analgesics with reduced side effects.
Keywords: G-protein-biased agonist; analgesic; opioid peptide; rubiscolins; δ opioid receptor.
Conflict of interest statement
The principal author and one coauthor (M.Y.) are employees of a pharmaceutical company (Viatris Pharmaceuticals Japan Inc. and Pfizer Japan Inc., respectively). However, the present study has no financial or other relationships with these companies, as it was entirely sponsored by and performed at the Jikei University School of Medicine, National Cancer Center Research Institute, and Juntendo University Graduate School of Medicine.
Figures



References
-
- Khademi H., Kamangar F., Brennan P., Malekzadeh R. Opioid Therapy and its Side Effects: A Review. Arch. Iran. Med. 2016;19:870–876. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

