Possibility of underestimation of COVID-19 prevalence by PCR and serological tests
- PMID: 34642099
- PMCID: PMC8479325
- DOI: 10.1016/j.jmii.2021.09.005
Possibility of underestimation of COVID-19 prevalence by PCR and serological tests
Abstract
Background: Exact comprehension of the prevalence of SARS-CoV-2 infection is essential for the preventive measures. In the clinical settings, however, patients infected with SARS-CoV-2 may not be fully detected by PCR. In the long-term prevalence study, cut-off of IgG assay may not be appropriate due to waning IgG titer.
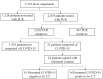
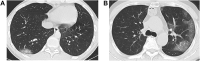
Methods: 24 PCR-negative subjects suspected of COVID-19 were categorized into cohorts termed "presumed COVID-19 positive" and "presumed COVID-19 negative" by chest CT images. IgG against nucleocapsid protein of SARS-CoV-2 (IgG (N)) and IgG against receptor biding domain of SARS-CoV-2 (IgG (RBD)) were measured in sera of the subjects and the concordance with the cohort categorization was assessed by receiver operating characteristics (ROC) analyses.
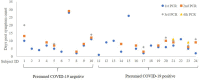
Results: Area under the curves (AUC's) by the ROC analyses with the 24 subjects were 0.982 with IgG (N) and 0.854 with IgG (RBD). Even when we excluded the subjects whose initial PCR was performed after five days from symptom onset, the AUC's were 0.967 with IgG (N) and 0.800 with IgG (RBD). The ROC analysis indicated 0.2 S/C as the optimum cut-off forIgG (N).
Conclusion: Both IgG (N) and IgG (RBD) titers were significantly elevated in subjects whose PCR never showed positive but suggestive of SARS-CoV-2 infection, which indicated the necessity of serological tests in complementing the shortcomings of PCR. For a long-term prevalence study, a cut-off lower than the one used in the ongoing infection phase (e.g. 0.2 S/C vs. 1.4 S/C) was indicated to be more appropriate for IgG (N).
Keywords: COVID-19; IgG; PCR; Prevalence; SARS-CoV-2; Serological test.
Copyright © 2021. Published by Elsevier B.V.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

