Single-Cell Atlas of Lineage States, Tumor Microenvironment, and Subtype-Specific Expression Programs in Gastric Cancer
- PMID: 34642171
- PMCID: PMC9394383
- DOI: 10.1158/2159-8290.CD-21-0683
Single-Cell Atlas of Lineage States, Tumor Microenvironment, and Subtype-Specific Expression Programs in Gastric Cancer
Abstract
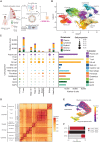
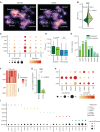
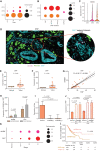
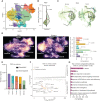
Gastric cancer heterogeneity represents a barrier to disease management. We generated a comprehensive single-cell atlas of gastric cancer (>200,000 cells) comprising 48 samples from 31 patients across clinical stages and histologic subtypes. We identified 34 distinct cell-lineage states including novel rare cell populations. Many lineage states exhibited distinct cancer-associated expression profiles, individually contributing to a combined tumor-wide molecular collage. We observed increased plasma cell proportions in diffuse-type tumors associated with epithelial-resident KLF2 and stage-wise accrual of cancer-associated fibroblast subpopulations marked by high INHBA and FAP coexpression. Single-cell comparisons between patient-derived organoids (PDO) and primary tumors highlighted inter- and intralineage similarities and differences, demarcating molecular boundaries of PDOs as experimental models. We complemented these findings by spatial transcriptomics, orthogonal validation in independent bulk RNA-sequencing cohorts, and functional demonstration using in vitro and in vivo models. Our results provide a high-resolution molecular resource of intra- and interpatient lineage states across distinct gastric cancer subtypes.
Significance: We profiled gastric malignancies at single-cell resolution and identified increased plasma cell proportions as a novel feature of diffuse-type tumors. We also uncovered distinct cancer-associated fibroblast subtypes with INHBA-FAP-high cell populations as predictors of poor clinical prognosis. Our findings highlight potential origins of deregulated cell states in the gastric tumor ecosystem. This article is highlighted in the In This Issue feature, p. 587.
©2021 The Authors; Published by the American Association for Cancer Research.
Figures


Comment in
- Cancer Discov. 12:587.
- Cancer Discov. 12:587.
References
-
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394–424. - PubMed
-
- Cristescu R, Lee J, Nebozhyn M, Kim KM, Ting JC, Wong SSet al. . Molecular analysis of gastric cancer identifies subtypes associated with distinct clinical outcomes. Nat Med 2015;21:449–56. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

