Contralateral Projection of Anterior Cingulate Cortex Contributes to Mirror-Image Pain
- PMID: 34642215
- PMCID: PMC8638682
- DOI: 10.1523/JNEUROSCI.0881-21.2021
Contralateral Projection of Anterior Cingulate Cortex Contributes to Mirror-Image Pain
Abstract
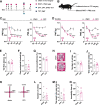
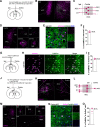
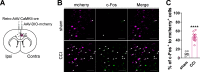
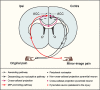
Long-term limb nerve injury often leads to mirror-image pain (MIP), an abnormal pain sensation in the limb contralateral to the injury. Although it is clear that MIP is mediated in part by central nociception processing, the underlying mechanisms remain poorly understood. The anterior cingulate cortex (ACC) is a key brain region that receives relayed peripheral nociceptive information from the contralateral limb. In this study, we induced MIP in male mice, in which a unilateral chronic constrictive injury of the sciatic nerve (CCI) induced a decreased nociceptive threshold in both hind limbs and an increased number of c-Fos-expressing neurons in the ACC both contralateral and ipsilateral to the injured limb. Using viral-mediated projection mapping, we observed that a portion of ACC neurons formed monosynaptic connections with contralateral ACC neurons. Furthermore, the number of cross-callosal projection ACC neurons that exhibited c-Fos signal was increased in MIP-expressing mice, suggesting enhanced transmission between ACC neurons of the two hemispheres. Moreover, selective inhibition of the cross-callosal projection ACC neurons contralateral to the injured limb normalized the nociceptive sensation of the uninjured limb without affecting the increased nociceptive sensation of the injured limb in CCI mice. In contrast, inhibition of the non-cross-callosal projection ACC neurons contralateral to the injury normalized the nociceptive sensation of the injured limb without affecting the MIP exhibited in the uninjured limb. These results reveal a circuit mechanism, namely, the cross-callosal projection of ACC between two hemispheres, that contributes to MIP and possibly other forms of contralateral migration of pain sensation.SIGNIFICANCE STATEMENT Mirror-image pain (MIP) refers to the increased pain sensitivity of the contralateral body part in patients with chronic pain. This pathology requires central processing, yet the mechanisms are less known. Here, we demonstrate that the cross-callosal projection neurons in the anterior cingulate cortex (ACC) contralateral to the injury contribute to MIP exhibited in the uninjured limb, but do not affect nociceptive sensation of the injured limb. In contrast, the non-cross-callosal projection neurons in the ACC contralateral to the injury contribute to nociceptive sensation of the injured limb, but do not affect MIP exhibited in the uninjured limb. Our study depicts a novel cross-callosal projection of ACC that contributes to MIP, providing a central mechanism for MIP in chronic pain state.
Keywords: anterior cingulate cortex; chronic constrictive injury of the sciatic nerve; excitatory pyramidal neuron; mirror-image pain; projection.
Copyright © 2021 the authors.
Figures






Similar articles
-
Nerve injury-induced neuropathic pain causes disinhibition of the anterior cingulate cortex.J Neurosci. 2014 Apr 23;34(17):5754-64. doi: 10.1523/JNEUROSCI.3667-13.2014. J Neurosci. 2014. PMID: 24760836 Free PMC article.
-
Neuropathic pain generates silent synapses in thalamic projection to anterior cingulate cortex.Pain. 2021 May 1;162(5):1322-1333. doi: 10.1097/j.pain.0000000000002149. Pain. 2021. PMID: 33230002
-
Upregulation of tumor necrosis factor-alpha in the anterior cingulate cortex contributes to neuropathic pain and pain-associated aversion.Neurobiol Dis. 2019 Oct;130:104456. doi: 10.1016/j.nbd.2019.04.012. Epub 2019 Apr 24. Neurobiol Dis. 2019. PMID: 31028871
-
Short-term synaptic plasticity in the nociceptive thalamic-anterior cingulate pathway.Mol Pain. 2009 Sep 4;5:51. doi: 10.1186/1744-8069-5-51. Mol Pain. 2009. PMID: 19732417 Free PMC article. Review.
-
Neuronal and microglial mechanisms for neuropathic pain in the spinal dorsal horn and anterior cingulate cortex.J Neurochem. 2017 May;141(4):486-498. doi: 10.1111/jnc.14001. Epub 2017 Mar 27. J Neurochem. 2017. PMID: 28251660 Review.
Cited by
-
Pain hypersensitivity in a pharmacological mouse model of attention-deficit/hyperactivity disorder.Proc Natl Acad Sci U S A. 2022 Jul 26;119(30):e2114094119. doi: 10.1073/pnas.2114094119. Epub 2022 Jul 19. Proc Natl Acad Sci U S A. 2022. PMID: 35858441 Free PMC article.
-
Segmental Upregulation of ASIC1 Channels in the Formalin Acute Pain Mouse Model.Pharmaceuticals (Basel). 2022 Dec 12;15(12):1539. doi: 10.3390/ph15121539. Pharmaceuticals (Basel). 2022. PMID: 36558990 Free PMC article.
-
Microglial Depletion does not Affect the Laterality of Mechanical Allodynia in Mice.Neurosci Bull. 2023 Aug;39(8):1229-1245. doi: 10.1007/s12264-022-01017-2. Epub 2023 Jan 13. Neurosci Bull. 2023. PMID: 36637789 Free PMC article.
-
Anterior cingulate cross-hemispheric inhibition via the claustrum resolves painful sensory conflict.Commun Biol. 2024 Mar 15;7(1):330. doi: 10.1038/s42003-024-06008-9. Commun Biol. 2024. PMID: 38491200 Free PMC article.
-
Acute and chronic stress differentially regulate pain via distinct ensembles in the paraventricular nucleus of hypothalamus.Mol Psychiatry. 2025 Aug 8. doi: 10.1038/s41380-025-03144-4. Online ahead of print. Mol Psychiatry. 2025. PMID: 40781546
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
