Emerging role of tumor-derived extracellular vesicles in T cell suppression and dysfunction in the tumor microenvironment
- PMID: 34642246
- PMCID: PMC8513270
- DOI: 10.1136/jitc-2021-003217
Emerging role of tumor-derived extracellular vesicles in T cell suppression and dysfunction in the tumor microenvironment
Abstract
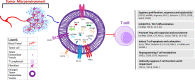
Immunotherapeutic drugs including immune checkpoint blockade antibodies have been approved to treat patients in many types of cancers. However, some patients have little or no reaction to the immunotherapy drugs. The mechanisms underlying resistance to tumor immunotherapy are complicated and involve multiple aspects, including tumor-intrinsic factors, formation of immunosuppressive microenvironment, and alteration of tumor and stromal cell metabolism in the tumor microenvironment. T cell is critical and participates in every aspect of antitumor response, and T cell dysfunction is a severe barrier for effective immunotherapy for cancer. Emerging evidence indicates that extracellular vesicles (EVs) secreted by tumor is one of the major factors that can induce T cell dysfunction. Tumor-derived EVs are widely distributed in serum, tissues, and the tumor microenvironment of patients with cancer, which serve as important communication vehicles for cancer cells. In addition, tumor-derived EVs can carry a variety of immune suppressive signals driving T cell dysfunction for tumor immunity. In this review, we explore the potential mechanisms employed by tumor-derived EVs to control T cell development and effector function within the tumor microenvironment. Especially, we focus on current understanding of how tumor-derived EVs molecularly and metabolically reprogram T cell fates and functions for tumor immunity. In addition, we discuss potential translations of targeting tumor-derived EVs to reconstitute suppressive tumor microenvironment or to develop antigen-based vaccines and drug delivery systems for cancer immunotherapy.
Keywords: adaptive immunity; immune evation; immune tolerance; immunotherapy; tumor microenvironment.
© Author(s) (or their employer(s)) 2021. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: No, there are no competing interests.
Figures
Similar articles
-
Immune Cell-Derived Extracellular Vesicles - New Strategies in Cancer Immunotherapy.Front Immunol. 2021 Dec 8;12:771551. doi: 10.3389/fimmu.2021.771551. eCollection 2021. Front Immunol. 2021. PMID: 34956197 Free PMC article. Review.
-
Tumor-Secreted Extracellular Vesicles Regulate T-Cell Costimulation and Can Be Manipulated To Induce Tumor-Specific T-Cell Responses.Gastroenterology. 2021 Aug;161(2):560-574.e11. doi: 10.1053/j.gastro.2021.04.036. Epub 2021 Apr 23. Gastroenterology. 2021. PMID: 33895168
-
Prospect of extracellular vesicles in tumor immunotherapy.Front Immunol. 2025 Feb 26;16:1525052. doi: 10.3389/fimmu.2025.1525052. eCollection 2025. Front Immunol. 2025. PMID: 40078996 Free PMC article. Review.
-
Harnessing extracellular vesicle-mediated crosstalk between T cells and cancer cells for therapeutic applications.J Control Release. 2025 Feb 10;378:266-280. doi: 10.1016/j.jconrel.2024.12.011. Epub 2024 Dec 16. J Control Release. 2025. PMID: 39657892 Review.
-
The Yin and Yang of tumour-derived extracellular vesicles in tumour immunity.J Biochem. 2021 Mar 5;169(2):155-161. doi: 10.1093/jb/mvaa132. J Biochem. 2021. PMID: 33226400
Cited by
-
Immune-regulating extracellular vesicles: a new frontier in autoimmune disease therapy.Essays Biochem. 2025 May 13:EBC20253016. doi: 10.1042/EBC20253016. Online ahead of print. Essays Biochem. 2025. PMID: 40366303 Free PMC article.
-
A Paradigm Shift in SSTI Management: The Multifunctional Role of Extracellular Vesicles.Int J Mol Sci. 2025 Jul 5;26(13):6481. doi: 10.3390/ijms26136481. Int J Mol Sci. 2025. PMID: 40650257 Free PMC article. Review.
-
Estrogens, Cancer and Immunity.Cancers (Basel). 2022 Apr 30;14(9):2265. doi: 10.3390/cancers14092265. Cancers (Basel). 2022. PMID: 35565393 Free PMC article. Review.
-
Exploring the Immunomodulatory Potential of Pancreatic Cancer-Derived Extracellular Vesicles through Proteomic and Functional Analyses.Cancers (Basel). 2024 May 8;16(10):1795. doi: 10.3390/cancers16101795. Cancers (Basel). 2024. PMID: 38791876 Free PMC article.
-
Detection of mitochondrial DNA mutations in circulating mitochondria-originated extracellular vesicles for potential diagnostic applications in pancreatic adenocarcinoma.Sci Rep. 2022 Nov 2;12(1):18455. doi: 10.1038/s41598-022-22006-5. Sci Rep. 2022. PMID: 36323735 Free PMC article.
References
-
- Théry C, Witwer KW, Aikawa E, et al. . Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for extracellular vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles 2018;7:1535750. 10.1080/20013078.2018.1535750 - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
