Pyrvinium pamoate regulates MGMT expression through suppressing the Wnt/β-catenin signaling pathway to enhance the glioblastoma sensitivity to temozolomide
- PMID: 34642308
- PMCID: PMC8511032
- DOI: 10.1038/s41420-021-00654-2
Pyrvinium pamoate regulates MGMT expression through suppressing the Wnt/β-catenin signaling pathway to enhance the glioblastoma sensitivity to temozolomide
Abstract
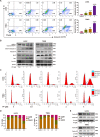
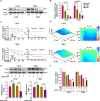
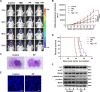
Temozolomide (TMZ) is the mainstream chemotherapeutic drug for treating glioblastoma multiforme (GBM), but the intrinsic or acquired chemoresistance to TMZ has become the leading clinical concern, which is related to the repair of DNA alkylation sites by O6-methylguanine-DNA methyltransferase (MGMT). Pyrvinium pamoate (PP), the FDA-approved anthelminthic drug, has been reported to inhibit the Wnt/β-catenin pathway within numerous cancer types, and Wnt/β-catenin signaling pathway can modulate the expression of MGMT gene. However, whether PP affects the expression of MGMT and enhances TMZ sensitivity in GBM cells remains unclear. In the present study, we found that PP and TMZ had synergistic effect on inhibiting the viability of GBM cells, and PP induced inhibition of MGMT and enhanced the TMZ chemosensitivity of GBM cells through down-regulating Wnt/β-catenin pathway. Moreover, the overexpression of MGMT or β-catenin weakened the synergy between PP and TMZ. The mechanism of PP in inhibiting the Wnt pathway was indicated that PP resulted in the degradation of β-catenin via the AKT/GSK3β/β-catenin signaling axis. Moreover, Ser552 phosphorylation in β-catenin, which promotes its nuclear accumulation and transcriptional activity, is blocked by PP that also inhibits the Wnt pathway to some extent. The intracranial GBM mouse model also demonstrated that the synergy between PP and TMZ could be achieved through down-regulating β-catenin and MGMT, which prolonged the survival time of tumor-bearing mice. Taken together, our data suggest that PP may serve as the prospect medicine to improve the chemotherapeutic effect on GBM, especially for chemoresistant to TMZ induced by MGMT overexpression.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




Similar articles
-
Overview of Wnt/β-Catenin Pathway and DNA Damage/Repair in Cancer.Biology (Basel). 2025 Feb 11;14(2):185. doi: 10.3390/biology14020185. Biology (Basel). 2025. PMID: 40001953 Free PMC article. Review.
-
β-catenin contributes to cordycepin-induced MGMT inhibition and reduction of temozolomide resistance in glioma cells by increasing intracellular reactive oxygen species.Cancer Lett. 2018 Oct 28;435:66-79. doi: 10.1016/j.canlet.2018.07.040. Epub 2018 Aug 4. Cancer Lett. 2018. PMID: 30081068
-
20(S)-ginsenoside-Rg3 reverses temozolomide resistance and restrains epithelial-mesenchymal transition progression in glioblastoma.Cancer Sci. 2019 Jan;110(1):389-400. doi: 10.1111/cas.13881. Epub 2018 Dec 14. Cancer Sci. 2019. PMID: 30431207 Free PMC article.
-
NBM-BMX, an HDAC8 Inhibitor, Overcomes Temozolomide Resistance in Glioblastoma Multiforme by Downregulating the β-Catenin/c-Myc/SOX2 Pathway and Upregulating p53-Mediated MGMT Inhibition.Int J Mol Sci. 2021 May 31;22(11):5907. doi: 10.3390/ijms22115907. Int J Mol Sci. 2021. PMID: 34072831 Free PMC article.
-
Temozolomide resistance in glioblastoma multiforme.Genes Dis. 2016 May 11;3(3):198-210. doi: 10.1016/j.gendis.2016.04.007. eCollection 2016 Sep. Genes Dis. 2016. PMID: 30258889 Free PMC article. Review.
Cited by
-
Accumulated β-catenin is associated with human atrial fibrosis and atrial fibrillation.J Transl Med. 2024 Aug 5;22(1):734. doi: 10.1186/s12967-024-05558-0. J Transl Med. 2024. PMID: 39103891 Free PMC article.
-
Wnt Signaling and Therapeutic Resistance in Castration-Resistant Prostate Cancer.Curr Pharmacol Rep. 2023 Oct;9(5):261-274. doi: 10.1007/s40495-023-00333-z. Epub 2023 Sep 19. Curr Pharmacol Rep. 2023. PMID: 37994344 Free PMC article.
-
Overview of Wnt/β-Catenin Pathway and DNA Damage/Repair in Cancer.Biology (Basel). 2025 Feb 11;14(2):185. doi: 10.3390/biology14020185. Biology (Basel). 2025. PMID: 40001953 Free PMC article. Review.
-
Live-cell imaging in human colonic monolayers reveals ERK waves limit the stem cell compartment to maintain epithelial homeostasis.Elife. 2022 Sep 12;11:e78837. doi: 10.7554/eLife.78837. Elife. 2022. PMID: 36094159 Free PMC article.
-
Chrysomycin A Inhibits the Proliferation, Migration and Invasion of U251 and U87-MG Glioblastoma Cells to Exert Its Anti-Cancer Effects.Molecules. 2022 Sep 20;27(19):6148. doi: 10.3390/molecules27196148. Molecules. 2022. PMID: 36234681 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

